Researchers at MIT and in China have improved the old-fashioned solar still with a new inexpensive device that harnesses the sun to remove salt from water. Traditionally, these kinds of systems use a wick to draw water, but once the wick becomes fouled with salt, the device needs cleaning or other maintenance. Not exactly what you want in a survival situation. You can read the paper in Nature if you want more details.
The key to this new technique is black paint and polyurethane with 2.5-millimeter holes drilled in it. The idea is that warmer water above the insulating medium causes the salt to concentrate in the cooler water beneath the insulator allowing efficient vaporization of the water. As the water evaporates, it causes the salt concentration at the top to rise, which then sinks due to the higher density and lower-concentration salt water rises to the top to evaporate.
Because the materials are commonplace, the team says a one-meter-square system costs about $4 to produce. A system that size could provide a family’s daily drinking water.
So far, the prototype system has worked in the lab for at least a week without accumulating salt. The next challenge is to scale it to something more practical, but due to the low cost and simplicity of the system, it seems it would be easy enough to make that happen or to reproduce the device for your own testing.
Desalination is a problem you can approach from many different angles. You can also harvest clean water from fog, something else that started at MIT.

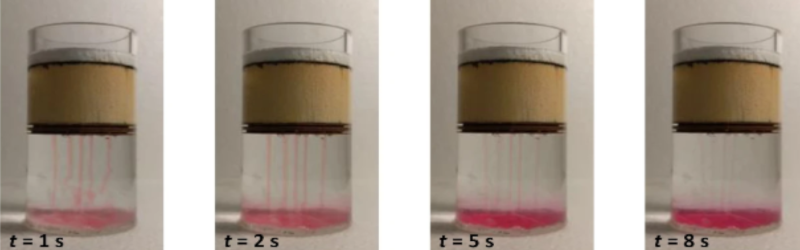














The description wasn’t very clear to me, but if I understand correctly, the idea is that the water is still evaporated, so it’s basically a solar still, but without the problem of salt crystals depositing in the evaporation vessel.
The warm water can dissolve more salt, which increased the density (apparently much more then the higher temperature decreases the density), which makes it flow down, taking the excess salt with it, preventing this salt from depositing on the surfaces of the device.
Is this really such a huge problem in solar stills? I’d think just letting the water evaporate and scraping the salt off at the end of the day could work (if the vessel is scrape-resistant), or simply changing the water a bit more often. As long as it’s warm, the salt should not crystalize, unless you allow to much of the water to evaporate. Changing the water more often does waste some energy because you’re replacing warm water with cold water, but this device does exactly the same, as far as I can tell.
I guess the main advantage is that you’re only heating a thin layer of water, making it more efficient?
The article could be greatly improved with a clear problem statement at the very beginning.
warm water can dissolve more salt, Uh, no.
Warm water does not ‘dissolve more salt’, at least not to any great degree. (Warm dissolves it *faster*, but the ultimate concentration of salt is not much different for warm vs cold water).
This looks like it’s encouraging stratification. It happens naturally in bigger ponds, but shallow ponds will allow the bottom to heat, encouraging convective mixing. This interposed layer frustrates that mixing.
Huh. I had to look that up. NaCl is sort of unique and you are correct it’s solubility does not change much with normal temperatures. Warm solutions absolutely dissolve most other solutes though than when colder. It’s how you make rock candy (sugar has a substantial increase of solubility with temperature) or do a fractional recrystalization.
And many things exhibit decreased solubility in warmer water. Oxygen is a pretty important one.
FWIW gases almost always have solubility decrease as a function of temperature, where solids almost always have solubility increase as a function of temperature.
@Paul
Uh, yes. At 0°C 366.5 g/l, at 100°C it’s 389.9 g/l, according to [1]. Solid salts tend to do this.
[1] https://en.wikipedia.org/wiki/Solubility_table
That difference is a lot smaller than I expected, but it’s obviously enough to sufficiently change the density for this to work.
Still no. You’re talking about an *enormous* temperature gradient for a *very small* solubility change.
More precisely, it’s a 6% change in solubility for a 37% change in temperature.
You’re being pedantic. 380 g/L +/- 3% qualifies as ” not much different for warm vs cold “.
Will it work on a small boat or survival raft etc, or will the incessant bobbing and rolling around on top of the worlds supply of not yet potable water mess up the fluid mechanics?
It’ll stir it up, and then we’re back to square one with deposits.
Hmmm yeah, that’s about what I thought, wonder what slosh protection like a bundle of semi permeable straws would do for it.
one could just hang it on a string?
I love these practical solutions below for this important problem :)
At a guess, evaporation of fresher (less salty) water is going to take less heat than evaporation of brine, so you’re getting additional efficiency that way, too.
I wonder if it could be used with calcium chloride to make a solar powered dehumidifier.
Yeah, I was mentally bookmarking it for a deeper dive into the science for control of solutions for other purposes. Glaubers salt for thermal transfer, liquid battery electrolytes etc etc.
However for that particular idea, I think it would work maybe to get an efficient first/last stage (Depending on which way round you’re looking at it), but may require multiple stages with other techniques.
How about we SOLVE thirst first?
Mine’s a pint, cheers!
Bio-engineer people to be more like desert animals.
How about you recognise that millions of activities can progress in parallel.
Liquid and gel dessicants are currently the most efficient way to extract moisture from the air.
Are they? Let’s see your tabletop dessicant regenerator then.
Lots of people here not reading the paper. It’s actually quite simple:
– Heating a thin layer of water is more efficient than heating your entire tank of water, because you can start generating vapour nearly immediately rather than waiting for the entire tank to boil (which if it takes longer than daylight is available for will never occur).
– Heating a thin layer to beyond saturation means salt crystals are left behind
– Salt left behind blocks transport of additional water (e.g. wicking)
– The perforated insulator allows the denser more saline partially evaporated water to flow back down via convection before it has evaporated beyond its saturation point (just needs to be more saturated than the bulk volume)
– The perforation size is chosen so the recirculation flow due to salinity is greater than the recirculation flow due to temperature difference, so the upper thin volume remains insulated and thermal losses are minimised (or in other words, saturated fluid flows back down, but unsaturated fluids remains to receive additional heating and release the maximum quantity of vapour)
– Because the fluid in the evaporator section never reaches saturation, no salt is deposited
Thank you. The process makes more sense now. This does make it a strong improvement on a solar still.
That really depends, as one thing that is great about some solar still designs is they also produce salt, two products at once may trump the water generation specialty process of this, at least for some use cases – this is still very interesting, but I’m not sure its a ‘strong improvement’ at all, more a valid alternative depending on your desires.
Looking through the paper:
the perforation diameter ~3mm
insulation thickness ~3cm
surface pool depth ~3mm
Thank you for the summary psudonymous! Excellent work.
Thanks for this. Their cost estimate is way off. You can’t even get a 1 square metre of polyurethane insulation 3cm thick for $4, not to mention the rest of the materials and the cost of building it.
Not at retail for pre-made sheet maybe, but if you were to manufacture these at scale you’d not bother buying pre-made foam and punching holes, and instead cast it to the size needed with the holes in place
China could do it for a 0ne Shiba!
thanks, that’s a great explanation.
That ^^ should have been in the HaD article… (maybe a bit more condensed but still)
“A thin layer of water on top of the insulator heats up, evaporates, it’s salinity increases and the now heavier solution flows thru the holes into the reservoir below, pushing up fresh water to repeat the cycle.”
I assume new water is added to the reservoir to keep the overall waterlevel level? ;-)
Or is it a kinda floating insulator – it’s specific weight would need to be right between the two water “types” (for lack of a better term).
But It would automatically indicate when it’s done: If there’s still water on top of the insulator it’s still running. If not (the insulator isn’t submerged anymore) the salinity has reached it’s allowed maximum level.
I disagree a little.
1. It isn’t convection per se, but saline density imbalance, that makes the concentrated saline sink. (After all, the top water is hot and would stay there otherwise)
2. The warm sinking saline can warm up the rising replacement water via a heat exchange. Now, I wonder if this happens naturally because of the hole size? Or will they mix too much?
Maybe therefore, as an improvement, put a couple of straws in each hole, so the heat can be exchanged, but with no mixing.
Convection doe snot result from just heat, it’s a flow pattern. Here, the pore size and separation are chosen to ensure the convection ‘loops’ effectively clear high concentration saline without excessive removal of low-concentration saline.
And China needs de-salinators for its Island Building (i.e. Empire Building) program.
The sneaky buggers… we should nuke the Dutch to let them know nobody gets away with that kind of thing. ;-)
That is a very smart design, however I think that some fluid dynamics simulations could improve it further by allowing them to optimise the design such that there were separate channels for upflowing water and downward flowing water, otherwise the device has to sort that out for itself and that would have a higher risk of turbulence and or oscillations. Perhaps the downward flowing tubes could be a bit longer, it may not take much to constrain the flow adequately, just enough to be on the right side of preventing the potential for chaos emerging in the system.
They already did that in the paper: the optimal pore size was found where flow self-organises (essentially captured Rayleigh–Bénard convection) to separate inflow and outflow at the macro scale.
I think the design works well in part due to these beneficial inefficiencies – if water circulated too well it’d keep cooling off the hot water at the top. Flow needs to be *just enough* and no more.
For all the science, it really lacks clarity on how the separated desalinated water is separated by evaporation and condensation, and why this is better than simply evaporating a pool of salt water itself.
Agreed. To me it looks like a clever way to make salty water more salty. How do they capture the pure water vapor? Oh, they condense it . . . . How? I don’t see that half of the equation. Did I miss it in the paper?
How do we collect the desalinated water ? Is it the surface water on the top of the system. Or is it the evaporated water collected with a serpentine ? Is the evaporated water lost ? It’s all but clear. The Nature paper doesn’t give a clue.
So it’s a floating evporating pan with small holes.
Of course that sounds a lot less fancy than “confined water layer”.. but I ain’t got time for that.
And that will ho right in the file draw with the high mileage carburetor.
They just want you to feel good not solve your problems.
Collecting water from fog did not start at MIT, they got the idea from people in Peru who use nets to trap water molecules in the air which then drip down into a reservoir.
There’s plenty of fog in their paper!
Guessing could benefit in like a desert situation possibly and concurrently maybe have some sort of production inside like a greenhouse above ground and the stratification layer would be at ground level or below with the system possibly benefiting from cooler below ground conditions?
Even if not this design exactly… reminds me of geothermal for greenhouses whether or not the Chinese greenhouse design with the northern slope earthen wall thicker than the front line most the height as well as the south face having earthen mound with some slightly below ground. That’s more colder conditions related… though I recall more than one time hearing about greenhouse owners complaining about gas prices for heating.
Therefore, wondering about a ratio of desalination greenhouse or desalination designs to utilize sea water more for farming versus well… just… seawater farming maybe? Though then floating desalination units above the farms might be a volume to consider? OK… farming to me some days seems almost stone age agrarian like when considering volume potential and aircraft with tetraethyl lead spewing only flying above who knows what?