There were news stories afoot this week with somewhat breathless headlines that suggested a medical breakthrough was at hand: “In a 1st, two people receive transfusions of lab-grown blood cells.” A headline like that certainly catches the eye, especially as the holidays approach and the inevitable calls for increased blood donations that always seem to happen this time of year as the supply gets pinched. Does a headline like that mean that someone is working on completely artificial blood?
As always with this sort of thing, the answer is a mixed bag. Yes, a team in the UK has transfused two patients with a small amount of lab-grown red blood cells, and it’s the first time that particular procedure has been performed. But while the headline is technically correct, the amount transfused was very small, so the day when lab-grown whole blood transfusions replace donated blood isn’t exactly here yet. But the details of what was done and why it was attempted are the really interesting part here, and it’s worth a deep dive because it does potentially point the way to a future where totally synthetic blood may be a real thing.
Growing Up Red
To understand what’s being done in this trial, which is called “Recovery and survival of stem cell originated red cells”, or RESTORE, we have to look into the process of blood formation in some detail. The journey from a single cell type to whole blood filled with a balance of red blood cells, white blood cells, platelets, and myriad other specialized cells and factors, is called hematopoiesis. It’s an immensely complex and tightly regulated process, but it all begins with the simplest and in some ways the most important cells in the body: stem cells, which are undifferentiated cells that can make an essentially unlimited number of copies of themselves.
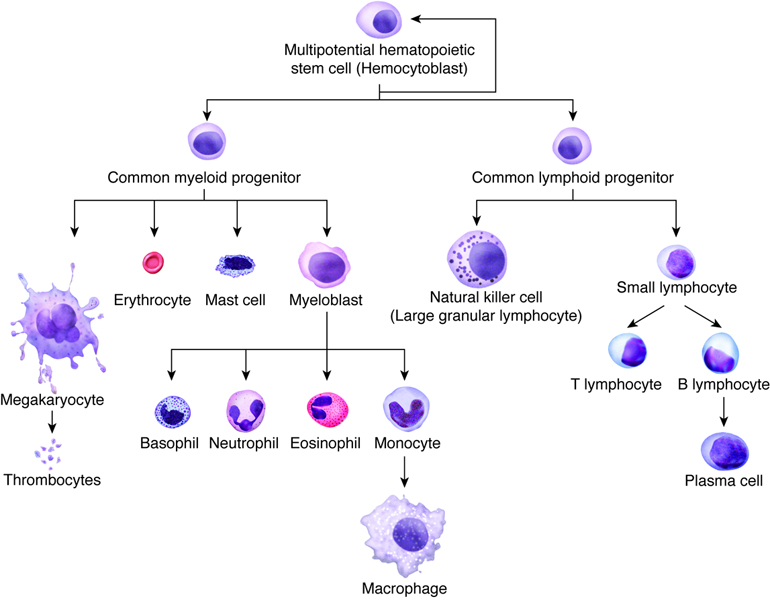
The stem cell at the root of hematopoiesis is called a hemocytoblast. In adults, hemocytoblasts are located mainly in the bone marrow, particularly in the sternum, the vertebral bodies, the ribs, and the wings of the pelvis bones. In response to the presence or absence of certain growth factors, hemocytoblasts undergo a series of divisions that result in increasingly differentiated cells with specialized functions. While some hemocytoblasts end up going down a branch that leads to the various types of cells that make up our immune system — the leukocytes, or white blood cells — others begin a process of differentiation into cells specialized for the transport of oxygen and carbon dioxide: the red blood cells (RBCs), also called erythrocytes.
In the process of differentiation, or erythropoiesis, the stem cells undergo a dramatic transformation in both size and shape. The developing red blood cells get smaller and start to take on their characteristic biconcave disc shape. Genes that code for heme proteins start to get expressed, and the developing erythrocytes start to turn red as the oxygen-carrying protein hemoglobin accumulates in the cytoplasm. Eventually, the nucleus that was present in the stem cell, which has been shrinking during the whole differentiation process, is ejected from the immature erythrocyte, leaving a small bag of hemoglobin and not much more.
The immature red blood cells at this stage are called reticulocytes. At this point they migrate from the marrow and into circulation, where they mature into erythrocytes in a couple of days. Reticulocytes make up about 1% of the RBCs in a healthy patient at any given time, with the other 99% being a mixed population of ages up to about four months. When they get that old the RBCs are too damaged to do their job, so they are removed from circulation and recycled by the spleen, with the elemental iron from their hemoglobin recycled for the next round of erythropoiesis.
Baby Blood Cells
In a healthy adult, erythropoiesis is a prodigiously productive process; even though it takes three weeks to go from stem cell to reticulocyte, the marrow puts something like 200 billion new RBCs into circulation every day. This ability to quickly rebuild our stock of RBCs is the key to blood donation; typically, blood donors completely recover from the donation of half a liter of whole blood within 20 days or so. As a result of this rapid recycling, blood donation has become an absolutely critical life-saving tool, used to treat a huge range of diseases and disorders.
But, as life-saving as whole blood transfusions may be, there can be complications. Red blood cells carry protein factors on their surface — the familiar “ABO” groupings — that can, even when carefully typed and cross-matched, eventually raise an immune reaction in the recipient. This tends to be most prevalent in frequent blood recipients, particularly in those with anemias like sickle cell anemia or thalassemia, or with clotting disorders like hemophilia.
One way to potentially get around the issue of developing what essentially amounts to a “blood allergy” is to increase the time between transfusions, and that’s exactly what the RESTORE trial is looking at. Rather than transfusing whole blood containing RBCs with a wide range of ages, they want to be able to transfuse patients with blood where every RBC is exactly the same age and brand new. That way, hypothetically at least, the transfused RBCs would survive for their full 120-day lifespan, rather than being retired continuously starting from nearly the moment of transfusion.
The first step in exploring how useful lab-grown blood is in treating diseases is to make some blood. While there hasn’t been a paper published from the RESTORE trial yet, in vitro erythropoiesis has been a pretty standard lab procedure for decades. Methods vary, but from the description given by the RESTORE team, it’s likely that they’re isolating and amplifying the small number of hematopoietic stem cells that circulate in the blood along with mature cells. These cells have antibodies on their surface that mature red blood cells lack, and that fact can be used to isolate them from the rest of the cells. A small population of stem cells can then be grown up in the appropriate growth medium.
To turn the stem cells into RBCs, the culture can be treated with erythropoietin, a protein that’s normally secreted by the kidneys. Erythropoietin, or EPO, is secreted when the body senses low blood oxygen; the body responds by stimulating the differentiation of stem cells into RBCs, to increase the oxygen-carrying capacity of the blood. EPO gained fame in the 1990s as a performance-enhancing drug when used by athletes, particularly cyclists, to increase the oxygen-carrying capacity of their blood.
For the RESTORE study, whole blood is obtained from healthy donors, stem cells are purified from the whole blood, and RBCs are cultured. Some of the whole blood is also set aside as a control. Both batches of blood are then labeled with a mildly radioactive tracer. On the donor side, healthy volunteers are given a very small transfusion — just a few milliliters — of the cultured blood. They’ll be followed over the next four months, with samples of their blood being analyzed to see how many of the cultured RBCs remain. After all the cultured blood has been cleared out, the experiment is repeated with the donated blood.
If all goes well, the RESTORE team will transfuse a total of ten volunteers. They expect that the cultured RBCs will last longer in circulation than the whole blood transfusion; if so, this may open the door to improved therapies for patients in need of frequent blood transfusions. There’s a lot of ground to cover before that, of course, not least of which is scaling up a method that can currently produce enough cultured RBCs for one person.
The Future of Synthetic Blood
But could a similar process one day result in completely lab-grown whole blood? Possibly, but whole blood is far more complex than just RBCs, and learning to grow large quantities of it is likely to be orders of magnitude more difficult. What would make this possible is the initial stem cell: the hemocytoblast. Since every cell in whole blood descends from that one cell type, it should be possible to grow whole blood completely in vitro. This doesn’t mean that the process would be entirely synthetic, of course. Those stem cells have to come from somewhere, and the most obvious source would be human donors. That begs the question of why you’d bother with the in vitro steps at all; if you’ve got to get a donation, just get whole blood and be done with it, right?
While that’s true, there would be significant benefits to turning donated stem cells into artificial whole blood. The main advantage is that since stem cells are essentially immortal, a single donation could potentially generate an unlimited amount of whole blood. This could be of great benefit anywhere the pool of potential blood donors is limited, but there still may be demand for blood in an emergency — think space travel. And even if generating whole blood from a stem cell culture never proves to be possible, being able to scale up erythrocyte production and mix it with donated plasma could be tremendously valuable — thanks to plasmapheresis, plasma can be donated much more often than whole blood.
The day when human whole blood donations are no longer needed will probably never come, and if it does it’s a long way off. But the fact that the RESTORE trial has managed to grow even the few milliliters of blood needed to do their initial experiments is exciting news. Not only might this trial result in tangible benefits to patients in need right now, but it may also open the door to unlimited whole blood on demand.















Can Androids donate artificial blood?
Only when they dream of electric sheep…
Neat article. RBC more precisely is “red blood corpuscles”… the RBC in the blood stream is not a cell…it has no nucleus.
RBCs FOR THE RBC GOD
Lymphocytes for Nurgle
Red corpuscles matter.
I’m feeling under the weather, I’ll get some new blood.
I did get a transfusion in 2019. Clear out the old. I wasn’t there at the time.
What?
Same…
I went to bed on a Friday, and as near as I can tell, ended up in the hospital four days later. The first week or so is very hazy. The transfusion was apparently gecause they were trying to figure what was wrong, or because my blood was so lumpy. I got it third hand after the fact.
I know what this is like.
Oh great. Now please a miniaturized version of the blood producing machine that can be installed in an hotel room, for sports events like Olympic Games and Tour de France
This was my thought as soon as I started reading this: pro bike racers are for sure going to be using brand new Day 0 blood. They (probably) already do complete blood replacement after every stage of the race but they get, as noted, normal blood with already damaged/aged red blood corpuscles.
I always wonder if the blood they drain out get put back into the donation banks. I hope it does: it’s perfectly good, with a soupcon of interesting drugs in it.
People using blood banks for vanity oughta be drained
At this rate it was always going to be inevitable to reach a point at which “natty” becomes too abstract to call. Really the only choice is to define and regulate juicing, get it above board.
Why do that when you can likely get a better athletic boost by using gene therapy to pull in the Tibetan Sherpas’ phosphocreatine changes and the larger spleens, etc. from the Indonesian Bajau free divers.
Though admittedly you would likely need to start that at a younger age and results may vary.
There is already a thing called “Young Blood” which is similiar to this.
Should be called GMO blood.
Did I miss something about genetic modification? All I read was that they are cultured from blood-harvested stem cells.
Labeling things GMO that are neither GM nor O is stupid. Much as is fearing GMO as some sort of bad thing. If when in need you prefer to be allowed to die than to be infused with an improved form of blood transfusion that’s your call. If you don’t want to eat GMO food that is more plentiful or more nutritious that’s fine too. Don’t encourage others to make the same bad decision.
GMO is a bad thing. For a plethora of reasons. It’s not a good decision at all. You’re dead wrong on this one.
You have glyphosate and atrazine playing havoc with your endocrine system right this second, by the way. And GMO enabled this. But hey at least we can feed 16 billion people (which will lead us to multiply to that point and have an unprecedented mass die-off when we overshoot it)
ROFL… ummmm… no. No more feeding the silly GMO troll folks, nothing to see here!
Why?
You include things like the bacteria used to produce human insulin in that incredibly sweeping statement?
This is a stupid hot take. Genetic modification like any tool is neither good nor bad. Modifying for crops to resist an herbicide isn’t good but what about modifying crops to be more nutritious and drought resistant? What about modifying a tree to such up as much CO2 as possible as fast as possible?
Genetic modification is a tool and so are you.
You’re already eating foods that have been genetically modified through cross breeding.
If you eat anything made from corn you can thank a multi-generational genetic modification process of crossbreeding, likely starting from a long extinct giant grass to get to maize, then corn. Corn continues to be one of the most mucked about with plants to improve flavor and other desirable aspects.
It’s also very likely you eat some food that’s descended from plants that were mutated by being blasted with gamma radiation.
Look up radiation gardening. Plants are placed in circles around a bunker. Atop the bunker is a tower. Periodically a gamma radiation source is lifted from the bunker then up the tower.
When the plants put on flowers and fruit they’re checked for desirable mutations. Seeds from the desirable mutations are planted and raised to maturity to see if the mutation breeds true. If it does, that may be a new product.
Ruby red grapefruit originated in a radiation garden, but through many generations of trees the color has been fading back towards the pink it was originally. So it’s back to the radiation gardens to try and hit that same mutation again. Other attempts are being done by plant geneticists to directly alter the grapefruit genome to produce the dark red color and keep it from reverting.
Thank your for this comment. I knew about genetic modification by use of gamma radiation but I was totally ignorant of the concept of radiation gardens.
GMO in and of itself is not necessarily bad.
That said our current applications of it via Bayer / Syngenta, etc. does suck.
Imagine a future where blood, tissues and maybe even replacement organs are grown.
Imagine a future of very long duration space missions.
To improve the chances of success and survival they will probably want to grow some of this stuff on the ship to be ready in case an astronaut needs it.
Space, energy and weight carrying ability will still probably be limited. Whatever functions can be combined will be.
They will also need to grow food…..
If they are growing replacement parts, they are capable of providing food stock
Cannibals will be pleased.
People without guilt.
The short story (novella?) Nightingale by Alastair Reynolds takes place on a hospital space ship that’s considered neutral territory for both sides in a war.
I’ll try not to spoil anything but at one point the characters are swimming through a tank of medical fluid and keep bumping into curtains of “material”. Turns out the material was human skin grown to the size of a sheet of plywood for graft purposes. The creepy factor ramps up a tangential curve from there.
Anyway, creepy skin tanks aside, being able to grow spare parts on the fly from raw materials would be worth the payload penalties I think. Hell, being able to drop critical medical supplies from orbit to battlefield or disaster field hospitals would be pretty awesome too.
“Orbital med drop in bound, 500 liters of type-O, two minutes. Four pallets of tissue grafts to follow. Clear the LZ!”
The parts will end up with proprietary connectors.
That gives a whole new meaning to an old but good rule of thumb, “never date someone who buys Apple products”.
Blood is food
Does this lab grown rbcs belong to ABO blood grouping
Does the blood or stem cells collected from Donar with blood group ‘A’ can be used to grow only Blood group A?
I do not no answer but if they only make O neg, there are no* antigens and it is universal donor. *there’s a bunch of minor ones but those could easily be engineered off as well
This reminds me of past predictions about computers. Sure, it could take a century to perfect but that’s really not that long. Humans are such ephemeral creatures that give their own lifespans far too much significance.