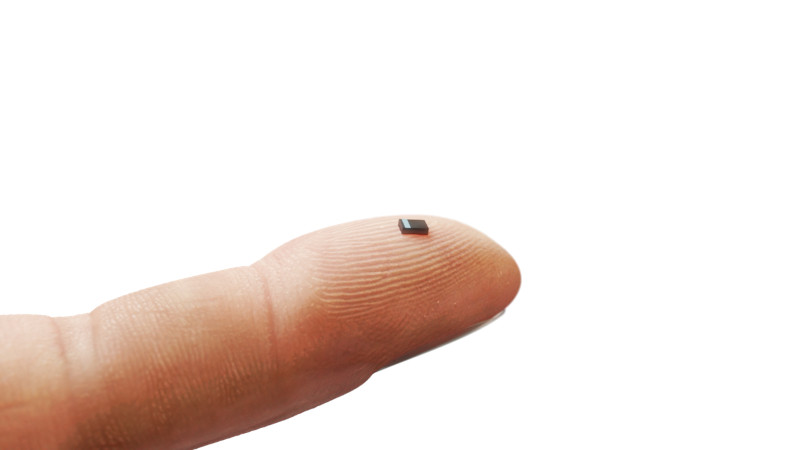
We’re used to some pretty small batteries in miniaturized electronics, thanks to the manufacture of lithium-polymer pouch cells. But they’re still pretty big, and they’re hardly the most stable power storage solution. The French company ITEN may have an answer for designers of micro-power devices though, in the form of a range of tiny surface-mount solid-state rechargeable lithium batteries. These come in a range of capacities from 0.1 mAh to 0.5 mAh, and in a 3.2 by 2.5 mm package look very much like any other slightly larger SMD chip component.
These devices are most likely to be found in applications such as remote wireless sensors, where they can store the energy from a small solar cell or similar to produce the burst of power required to transmit a packet of data as well as the tiny current required to keep things ticking over. The solid state chemistry should provide a long life and lack of leaks. For now they have some evaluation kits on offer, and unless we missed something, no full data sheet. We’d be particularly interested to learn about their temperature sensitivity when it comes to soldering, as we’ve taken to heart the warnings about soldering to more traditional lithium cells.
Via CNX Software.













How would these be attached then? Spot welding like regular battery packs are built?
silver epoxy glue, like microwave mixer diodes.
They’d be soldered just like any other SMD component. There’s no need for welds when the cell doesn’t hold enough energy to desolder itself.
The Henry is a unit of inductance, which has nothing to do with battery capacity. The linked press release refers to the capacity in uAh, so this article does have a typo, if the intention is to observe SI conventions on written units: https://en.m.wikipedia.org/wiki/International_System_of_Units
Shame? A little over-dramatic, perhaps?
It’s interesting that, in medical terminology, they decided to phase out names of body parts named from people.
The on-chip battery — the world’s smallest to date — still has a minimum energy density of 100 microwatt hours (μWh)/cm2. A micro origami process was applied to layer current collectors and electrode strips made of polymeric, metallic and dielectric materials were layered onto a tensioned wafer surface.
aint 500uAh /2.5V equal to 200uF capacitor?
Assuming the battery operates from 2.5V down to 2V, the capacitance needed for the same energy and voltage range is 1000uF.
You seem to forget these are solid-state batteries. They can operate in the normal -40C to 85C temperature range, so it’s likely they will work with your typical reflow process.
“Normal” for lithium batteries is 0-40 C. Solid state electrolytes don’t really work at all under freezing conditions or in many cases below room temperature, but they will survive it. That’s because of the low ionic conductivity of the material at lower temperatures. Many such batteries only really start working when they get hot.
From the website: “ITEN’s charter is precisely to develop, manufacture and sell fast-rechargeable solid-state micro-batteries in the form of Surface Mounted Devices (SMD) to be assembled and handled like any other SMD component by using pick-&-place and solder reflow techniques.”
Murata’s also been promising some “soon” for a while. They might be available to industrial customers, but I check in with them every once in a while and can’t find them for sale.
Maybe we should be using coulombs for the charge capacities at which these devices operate. Assuming I’ve done my calculations correct, 0.5mAh or 500µAh would be simply 1.8C.
Using coulomb avoids the need for SI prefixes, like m or µ, at these scales, but replaces the hour/Henry confusion with coulomb/Celsius ambiguity and a general lack of familiarity with coulomb as a unit of measure. At least there’s the degree symbol to help. I.e., its capacity is 1.8C, not 1.8ºC.
Iten, are you listening? Coulombs for the win!
Engineers in the future will curse you for splintering the standard in strange specific cases :)
There’s also the issue that C is used to represent the charge/discharge rate of a battery.
– There are only a few letters for everything under the sun. Then you have some Greek letters… We are bound to reuse them – said a teacher once to a student that confused Beta at the blackboard with the transistor gain.
– We still have the Gothic Manuscript Alphabet – said another student.
He was expelled from class before having a chance to explain that another teacher was actually using the Gothic Manuscript Alphabet for electromagnetism theory.
When you discharge a capacitor a constant current the voltage goes down linearly, while when discharging any kind of battery the voltage will be fairly constant.
The amount of *usable* energy in a capacitor highly depends on how you use it. E.g. when stabilizing a voltage source between 3.3 and 3.2 volts, you can only use ~10% of the total energy stored in the capacitor. When using a supercap with 2.5V and combine it with a Boost-converter able to operate down to 0.8V, you can use 90% of the stored energy. The total energy of a capacitor is W = 1/2 U^2 * C. Mind the “U^2” (and “C” refers to capacitance, i.e. Farad).
The Lithium battery with 2.5V and 100uAh stores about 0.9J (Joule). A 0.3F capacitor charged to 2.5V stores about the same amount of energy, as does a 1F capacitor charged to 1.35V. The small one has 0.8C (Coulomb) stored, the second one 1.35C.
Don’t mix Coulombs and Amperehours, these are different things.