Generally, when we talk about the production of hydrogen, the discussion is about either electrolysis of water into oxygen and hydrogen, or steam methane reforming (SMR). Although electrolysis is often mentioned – as it can create hydrogen using nothing but water and electricity – SMR is by far the most common source of hydrogen. Much of this is due to the low cost and high efficiency of SMR, but a major disadvantage of SMR is that large amounts of carbon dioxide are released, which offsets some of the benefits of using hydrogen as a fuel in the first place.
Although capturing this CO2 can be considered as a potential solution here, methane pyrolysis is a newer method that promises to offer the same benefits as SMR while also producing hydrogen and carbon, rather than CO2. With the many uses for hydrogen in industrial applications and other fields, such as the manufacturing of fertilizer, a direct replacement for SMR that produces green hydrogen would seem almost too good to be true.
What precisely is this methane pyrolysis, and what can be expect from it the coming years?
Carbon Waste
Methane (CH4) is most commonly found as the primary constituent of natural gas and is also prevalent as the output from methanogenesis, which includes the famous cow burps. With steam methane reforming and similar processes, the goal is to strip the hydrogen atoms from the single carbon atom, releasing the hydrogen for capturing. This leaves the carbon as essentially a waste product, that with SMR results in each carbon atom capturing two oxygen atoms to form carbon dioxide, our all too familiar greenhouse gas.
The basic reaction of SMR is given as:
CH4 + H2O ⇌ CO + 3 H2
This is an endothermic reaction, meaning that an SMR reactor is kept within a temperature range of approximately 800 – 900 °C in order for it to actually produce any significant amounts of hydrogen. The aforementioned CO2 shows up in in the additional Water-Gas Shift Reaction (WGSR), described as:
CO + H2O ⇌ CO2 + H2
The point of the WGSR is to extract additional hydrogen, increasing the overall efficiency of the SMR process. Additionally, catalysts are used to increase the efficiency of the reactions, resulting in an overall efficiency of SMR up to 75%.
The capital costs for an SMR installation are rather minor, with the continuing costs being primarily the natural gas feedstock and fuel for the heating of the reactor. If the produced carbon monoxide and carbon dioxide also have to be captured (so-called ‘blue’ hydrogen’), the capital and ongoing costs are going to be correspondingly higher and system efficiency lower (~60%). This makes carbon capture and storage with SMR economically unattractive, and is where ‘turquoise’ hydrogen produced using methane pyrolysis may make a lot of economical sense as well.
Turning Up The Heat
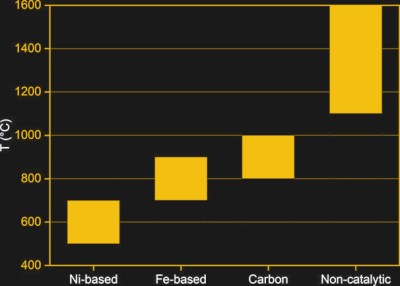
The main difference between SMR and methane pyrolysis is the temperature. It employs the thermal decomposition of methane, with temperatures of generally well above 900 °C, with some approaches explored over the decades suggesting up to 1,900 °C. A general issue with methane is that it is a very stable molecule, owing to its C-H bonds.
As a result of these strong bonds, thermal decomposition of methane without the presence of a catalyst generally won’t take place until a temperature of over 1,100 °C. The challenge over the past decades of research has been to find a suitable catalyst. Here metal catalysts have been mostly studied, including iron and nickel. A big issue with metal catalysts is their rapid deactivation through the formation of carbon deposits on the surface. Reactivating the catalyst by cleaning away the carbon deposit is problematic, and can result in the production of carbon dioxide.
Carbon catalysts have seen more research the past years, with a number of promising advantages making them rather appealing. Usually in the form of activated carbon and carbon black, their advantages can be summarized relative to metal catalysts as follows:
- Lower cost
- Better (thermal) stability
- Tolerance to impurities (e.g. sulfur in the natural gas)
- No need to regenerate the catalyst
- End product is pure carbon, without metals
- The catalyzed carbon deposit may still act as catalyst
A final, and very different, approach involves molten metals and salts, both in liquid bubble column reactors. In these columns of molten materials, the methane is introduced at the bottom, where the methane thermally decomposes inside the bubbles of natural gas. Once these bubbles reach the surface of the column, the hydrogen and carbon are released, with the lighter hydrogen separating from the carbon that remains suspended on top of the molten salt or metal.
These bubble column reactors would provide a rather ideal, continuous process where the carbon can be scooped off the surface without contaminating the molten material or the produced hydrogen. Currently, however, the research is still ongoing to find the right kind of salt or metal that would work for such a reaction column that would also accept the high operating temperatures.
This means that for the moment, carbon catalysts seem to be the best way to produce hydrogen from methane with pyrolysis, except for the higher temperature range required.
Prime Time Prep
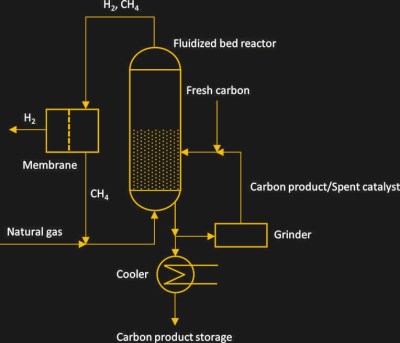
Although an advantage of methane pyrolysis is that today it can reach similar efficiencies as SMR with CCS, creating industrial-scale reactors that can run continuously without constant downtime for maintenance is still an ongoing challenge. A promising reactor type here is the fluidized-bed reactor, along with packed-bed reactors.
Despite the challenges with methane pyrolysis, it nevertheless would seem that its time has come. Today over 95% of hydrogen is produced with SMR. If the free release of carbon dioxide into the atmosphere is no longer acceptable, alternatives like methane pyrolysis have a fighting chance in the hydrogen production market. Of course there are many more ways to produce hydrogen, all with their own advantages and trade-offs.
A real concern is that many of these technologies require either a lot of electricity, or high temperatures. As noted by Sánchez-Bastardo et al., renewable energy is not likely to provide anywhere near the amount of electricity needed for electrolysis of water, even for just industrial demand. Simultaneously, thermal decomposition as with methane pyrolysis requires a source of thermal energy, which ultimately factors into the final cost picture and carbon footprint of the produced hydrogen.
This is where potentially Generation IV nuclear reactors may play a pivotal role, with the VHTR type (high-temperature, helium-cooled) providing an outlet temperature of 900 – 1,000 °C, which would be sufficient for methane pyrolysis. One such VHTR – China’s HTR-PM – is intended to be used for hydrogen production, in addition to electricity production.
One thing that does seem to be quite certain, however, is that hydrogen production will become a lot greener, with more and more carbon from methane scooped out of pyrolytic reactors rather than released into the atmosphere attached to oxygen atoms.
















Since you then do not release and use the energie stored in the Carbon , you must consider this as energieloss ,in that way it seems to me ,to be even less efficient than electrolysis.
But if you have a surplus of Methane , you need to get rid off , it might be the least harm full way ,off course.
That’s a very “investor” way to look at it, when in fact it’s rather an “incomplete utilization” of an energy source. You still get the major share of it through H2. It would serve to think of it in comparison with “burning it whole” plus “subsequent CO2 capture” (and then what, “storage” by injecting it into geological formations at high pressure?).
What’s more, CO2 sequestration is mostly O2 sequestration.
I can honestly say I’ve not thought of the consequences of losing oxygen as a result of pumping CO2 into the ground. Well spotted !! I could well imagine humanoids having to dig it up again in the future to get the oxygen back again.
Oh, we won’t run out of oxygen (it’s everywhere), but it means CO2 isn’t a very compact way to store carbon – it’ll be denser at those pressures than it is normally up here, but still, two of the three parts isn’t even carbon. Pure graphite is way more compact and by comparison, it can almost just be piled up out of the way if no one needs it at the moment.
I’ll take that excess carbon. I’ll put it in my garden where it breaks up my clay and stores nutrients and water. It’s also a good growing medium for soil microbes and fungus.
I’ve already made a couple batches of this carbon (biochar) out of waste wood. But nobody has offered me any carbon credits.
The carbon residue is sold as carbon black and is quite pure. It’s used in everything from paint, to tires.
Do we have a shortage of carbon black now? I doubt it.
This process would produce mountains of carbon.
Yes, but you can use the vast amounts of natural gas available in a zero carbon way. And the carbon black that precipitates is a solid. Its much easier to deal with. You cab just leave it on farmland and it will improve the soil.
For me the only “reasonable” way to get C (from CO2) back into the earth is filling all the coal mines with (naturally) grown wood.
All this greenwashing with planting trees is BS as long as the trees stay in “circulation”…
This doesn’t quite work with the CO2 from gas and oil but still.
It would (wood?) be better to make charcoal from the wood first, which is much more stable. I was thinking similar, but grow it and bury it at sea.
Actually that is a idea with growing and sinking kelp. It grows 5x faster than wood.
Nuclear reactors ideally like a near constant load, it is difficult to rapidly change output power levels. So if this is used during the off hours when human activity is less, as a secondary or tertiary roll I would see that as beneficial. Means that the reactor could be bigger and bigger usually means more efficient.
“Nuclear reactors are known for their ability to provide constant power but their output can also be modified to meet certain grid demands.
Operators can reduce power output by limiting the amount of steam that goes through a turbine to create electricity, or they can use control systems to slow down the nuclear reaction in the reactor.
France has been doing this for years to match daily and seasonal power demands and reactors in the U.S. Northwest and Canada flexibly operate each spring to accommodate additional hydropower on the grid.
While there are no technical or safety-related impacts in operating power reactors this way, there are some limitations. Operators can’t flex power output as much toward the end of the fuel cycle and it takes a lot of planning, forecasting and time to decrease the power output.
New reactors could offer even greater operational flexibility in the near future. Some small modular reactor (SMR) plants being developed will use multiple units within a single plant to quickly provide power when and where it’s needed. Each power module could also work independently to ramp down power or produce other products simultaneously. ”
https://www.energy.gov/ne/articles/3-ways-nuclear-more-flexible-you-might-think
Maybe I’m missing something obvious here, IE instead of using nuclear power to produce heat to turn water into steam to run a turbine to generate electricity to then turn back into heat to power this process, we skip the turbine stage and use the heat energy to directly power the methane pyrolysis reaction – Which, again, still uses methane, which will almost certainly come from natural gas, thus defeating the purpose entirely.
To the layman, if this is our excuse to approve new nuclear buildouts, wouldn’t make far more economic, thermodynamic, and economic sense just to use them to generate electricity, or, if we NEED hydrogen, for the electrolysis of water?
Not a politician or CEO, just some guy that cannot f$$$$$g stand all this “green fossil fuel” garbage. Hydrocarbon fuels are not inherently evil – digging them out of the ground and pretending they wont upset our current climate situation are problem.
I think the part you’re missing is that this process leaves you with solid carbon and hydrogen. No CO2 produced, and the hydrogen can be shipped off to use as a portable energy store wherever it would be most useful. The carbon can be literally piled up, dropped down a hole, etc.
This would provide transport fuel with no global warming downside (if you used biological sources of methane it could even be carbon negative)
My only assumption was that it would use “VHTR type (high-temperature, helium-cooled)”, nowhere did I suggest converting the thermal energy at the secondary loop into electricity. A load is not always electrical, it can be a thermal load, or a mechanical load.
The carbon can be a valuable product, and we’re better at getting energy into an electrical form very efficiently from hydrogen than we are at getting it from carbon, especially at smaller scales. If I remember correctly, if you minimize unnecessary heat loss, then even after you consider bond energy for the reaction, the hydrogen you get from methane pyrolysis will contain a little over half the energy of the original methane. However since we have hydrogen fuel cells, you’ll be able to retrieve more of that half of the energy than if it were just being combusted in an engine.
Though others can be more efficient, the solid oxide fuel cells that reform the fuel internally are very relevant. One problem is coking, which is addressed in this article, but they should also generate heat which while normally a loss of efficiency, would here at least help with the reforming.
Overall, this is much more efficient than electrolysis for the same reason that’s gotten us into this situation – it takes much less energy to take fuel out of the ground and use it, even at less than ideal efficiency, than it does to create the fuel from scratch using clean energy.
> carbon can be a valuable product
We have to admit though that (temporarily) using the carbon would amount to nothing more than a parlor trick with notes of government subsidies. For a net sequestration, the carbon must be permanently removed from the atmosphere. We cannot afford to turn this into yet another scam, which I think CCS is when it comes to CO2 – one cannot claim to “extract carbon from the atmosphere” when the only profit being made is from the production of goods that ultimately get “thermally utilized”.
There aren’t many high-value, long-term use scenarios either.
For example, carbon can be added to soil. It would also be nice to be able to use it to back-fill mining shafts or for land restoration. Unfortunately though, it appears that solid carbon from pyrolysis processes ends up having a comparatively low surface area per gram, making it less effective than activated carbon at immobilizing environmental pollutants from spills, mining waste, leachate and industrial discharge.
Not all pyrolysis processes are created equal, either. Molten metal and molten salt processes have non-zero loss rates, which also means the carbon is contaminated with metals or salts which are not easy to wash out and recover. From what I know, this sadly makes molten salt methane pyrolysis – derived carbon powder unsuitable for agricultural use.
Another process* (the development of which is funded by the German BMBF) is plasma pyrolysis. Here the carbon generated appears to be suitable for its addition to agricultural land, where it will stay for reasonably long periods of time.
In conclusion: Parting with the intellectual dishonesty that seems to permeate the economic reality of CCS, we need to focus on solid carbon use for sinks only. Usage scenarios where profit is being made with timely re-emission of CO2 will have to be discouraged.
* https://www.basf.com/gb/en/who-we-are/sustainability/we-produce-safely-and-efficiently/energy-and-climate-protection/carbon-management/interview-methane-pyrolysis.html
I suspect we’d find that a number of other uses which aren’t “thermal utilization” (nice euphemism) would find it useful and tolerate the levels of post-cleaning contamination we’d likely reach. This is a family of processes, after all, and it seems unlikely that every single one will result in carbon being intractably contaminated. As a random example, say it was pure iron alloying with the carbon that was the issue. Maybe we powder it, separate the majority of the iron from the majority of the carbon with corrosion, then recycle the portion that’s rust and carbon back into iron in place of a bit of scrap/ore. That leaves useful powdered carbon afterwards. Actually, if it was with acid that resembles the process you use to break apart the layers of graphite, IIRC.
The carbon left over would be valuable indeed. It would be burned at either the charcoal grill level or industrial coal fired plant level, either way marketed as “green” but producing atmospheric CO2 just the same, washing over the fact it started as a petrochemical in the first place.
Or it will be used in the brushes for motors that don’t warrant a brushless, as powdered lubrication, in pencil lead, etc
Carbon as a solid material is useful for oh so many things, and what comes out of this process as ‘waste’ will be ideal drop in replacement for many of these options…
Also a charcoal grill type replacement for actually cutting down tree… That isn’t a bad thing – sure it still ends up burnt in the end but you don’t have to turn the forest into an industrial farm just to make more stuff to burn. How beneficial that could work out with the energy requirements this process has isn’t going to be easy to calculate, but larger forests, with a wider range of tree age, more dead wood and undergrowth over the highly managed forest should be good for insects and capturing carbon at the same time. Burning carbon isn’t so evil it can’t possibly be allowed, it just has to be for a better reason than ‘we can’t be bothered to invest in energy infrastructure or efficiency’.
You’re missing the point of your own argument – the tree that I cut down to burn in my smoker contains a fixed amount of carbon. That tree took that much carbon out of the atmosphere, and burning it will return that much carbon to the atmosphere.
The carbon in the propane tank that powers my stove was trapped underground, outside of the system of the above mentioned atmosphere for eons. In releasing it into that atmosphere, the current balance is upset.
I can plant a tree to capture that carbon – but if it wasn’t released in the first place, I would not have to consider that upset to begin with. Further, lets imagine the life of that tree – it keeps growing, absorbs more carbon, and dies – that carbon is now no longer a fixed thing – regardless of what form it eventually takes, there is more net carbon in or available to said atmosphere simply due to the fact that it was taken out of an essentially inaccessible and non-interacting space (deep underground) and forced, one way or another, into our current atmosphere.
No I do see that point, however currently you can’t get away from fossil fuel entirely, it just isn’t possible for many years to come, and even when you can get away from the fuel part you will still have huge petrochemical industries extracting oil for plastics – Methane right now is more waste than product and the production of it isn’t going anywhere any time that soon.
This is a better option than just letting it out or simply burning it, as right now it produces hydrogen in bulk, allowing for less carbon intensive transit and industry and may prove to be a stepping stone that allows fully renewable hydrogen to become possible. All while also providing high quality carbon an important raw material in a useful form. In 200 years time this process isn’t likely to be widespread if done at all, as hopefully we haven’t all gone tribal and warlike where every local resource must be exploited hard to survive but remained a mostly co-operative global species all moving together towards being less destructive of our home.
And as the amount of carbon nature can sink is variable – the health of the ecosystem matters! If you turn everything into forestry plantations to get your burnables you loose so much more carbon sinking capacity. There is so much scope for more biomass in the ecosystems – a great deal of the long buried Carbon can be part of it. Also end up having to spend more energy on fertiliser and insecticides and the like nearby to make up for having such an out of balance ecosystem with thin probably quite acidic soil, which then goes on to make matters worse still as you are treating the largely self inflicted symptoms expensively rather than the causes…
> That tree took that much carbon out of the atmosphere, and burning it will return that much carbon to the atmosphere.
Trees take hundreds or thousands of years to grow and eventually decay – as long as you let them be. Trees burned for energy are cut down far sooner, in a couple decades.
The point: the amount of carbon going around the system is constant, but it is more concentrated in the biomass. When you cut down the biomass and release the carbon faster, the balance of the equilibrium changes towards more CO2 in the atmosphere. The less time the carbon spends in the form of a tree, the more time it spends in the form of CO2 in the atmosphere. That’s why, the use of biomass as an energy source is not climate neutral.
Don’t forget, hydrogen is used for a lot of things other than as an energy storage medium. We makes lots of H2 for ammonia production and other industrial uses, if we can remove the CO2 production by improving SMR, it will result in a huge net CO2 reduction.
I’m curious why the direct pyrolysis of water into hydrogen and oxygen isn’t getting more attention. Other people have gotten several thermochemical systems for doing this to at least function, but it doesn’t look like anyone is spending much time optimizing them, and that kind of surprises me. We have a LOT of water, and both of the outputs from this are desirable.
If you use pyrolysis on a fuel like this, you still avoid producing co2 but you also get much more energy out than you had to put in. We’re currently releasing lots of co2 to satisfy energy demands, so using a fuel without burning carbon is better than using water and continuing to burn carbon.
Calling this a green process is laughable. It uses fossil fuels as a feedstock. And even if it generates pure carbon as a byproduct and no CO2, they will probably sell that off as charcoal briquettes which get burned and generate… CO2.
I worked in fuels chemistry and catalysis before changing careers. This is another attempt to take a fossil fuels byproduct (methane) and make something more valuable / profitable from it. No more, no less.
Exactly. And you can assume the same for any “green” process that is publicized/advertised heavily. Same industrialists trying to sell a solution to the problem they made, making money on the way up and the way down (the solution will actually cause more problems that they can sell more “solutions” to later on)
Much like the anti-nuclear movement should have been an anti-corruption and pro-participation movement, competent criticism is of paramount importance and easily sabotaged. Globally, a lot more methane will be used, and I’d rather like to see methane-derived carbon being deposited in a solid form than pumping CO2 into the air, MWh / MWh.
Let this be an exercise in learning to demand accountability The alternative I see is that CO2 capture plants get the sweet government money, and subsequently sell the CO2 for polycarbonate production or similar. And when we find companies that claim to store it underground, I wouldn’t be surprised if we’ll end up finding out that it seeped back out over 10s of years, as afaik the reaction with basalt happens at a rather glacial rate unless the rock is ground into a powder and scattered on agricultural land.
If we didn’t produce CO2 and pollutants by burning fuels, the only issue would be eventually running out, which is a much better problem to have than the one we have now. And this process of pyrolysis isn’t specific to methane, it’s just very well suited to it since it’s the simplest alkane with the most hydrogen per carbon.
When we’re still mining coal to burn, and we’re flaring off methane instead of using it, I have a hard time seeing how this isn’t a greener option. Perfect seems to be the enemy of good. Even if someone decides to waste their high quality carbon by putting in extra effort to make imitation charcoal instead of a number of higher value non-fuel products, that just means they’ve replaced a different source of briquettes, which would have been burned anyway.
Not only that, but if you do want hydrogen, and you’re not wanting to be the reason someone can emit co2, then I guess you are stuck waiting until there’s enough power available during peak renewable hours that the grid is fully renewable, because otherwise no matter where you get your power, someone’s buying the fossil fuel power that you didn’t buy. Except, conventional power plants have to keep running because they can’t just stop and start like that, so that’s still not meeting your standard because someone always buys their power. To shut down the fossil fuel power plant you would have to provide enough no-co2 baseload or store enough power in peak supply hours to last the night. Maybe you can do that, but adding no-co2 baseload seems equal or better especially when we already know we need more grid supply overall. And this way is a source of energy outside of the grid.
:/
>Perfect seems to be the enemy of good.
Very true, the ‘greener than thou’ virtue signalling type stuff that makes many actually ready to go useful improvements ‘evil’ for not being enough. One more step towards a system that can perhaps work and not require a 10th century population and lifestyle isn’t allowed for not being green enough – “but we can’t possibly stop driving to our protests, or using the grid, the petrochemical everything etc – it would in be inhumanely inconvenient to do that.” So instead they shall sound like hypocrite for using oh so much ‘evil’ in protesting against a chance for a reduction in ‘evil’…
These sort of suggestions need watching to make sure the ‘promise’ of green credentials they show is actually realised, as it could easily turn into just another money making scheme that basically is the current petrochemical industry hiding behind a new marketing campaign. But that doesn’t mean such things shouldn’t be done – like all the complaints about a short term coal mine for premium grade coal to be used making steel in the UK – way way greener than going abroad for all the stuff and you can’t jump the whole industry from carbon fuel required to make the essential material for our society to other ‘green’ methods instantly…
Yes. Calling usage of non renewable fuels (methane, uranium) a green process is the only one and very poor hack which I see in this article.
It’s not “green” hydrogen, this is “blue”. That is, fossil and non-renewable in origin, but at least not CO2 releasing.
But you’re not wrong. This is a drive by the chem industry to minimize the ecological damage without losing their business. It’s a half measure.
Glass half full or half empty?
Although I only worked in the sector for a couple of years, I can assure you the petrochemical and fuels/catalysis industry has zero interest in anything other than a profit. If greenwashing a production method leads to more profit, that is the sole motivator. There is a huge debate about right/wrong of this and I’m personally not convinced either way, but the fact is chemical industry is a for-profit enterprise. This process in the article is describing reforming of waste/non-valuable byproducts and trying to condense them into something more useable AKA profitable. Outside of the technology to do so, the marketing of calling this “green” is horse-sh*t. The byproduct of this process, carbon, will be used for profit as well to the best of the company or industry’s ability. I don’t have the numbers but I’m pretty sure the demand for carbon for motor brushes (a reasonable use) or anything else is a very small fraction of the major use of fossil fuels (coke, coal, probably elemental carbon): combustion to produce energy. In any scenario, the tech to take a useless chemical and convert it to usable, profitable or energy efficient product(s) is basically the definition of petrochemical chemistry. But calling it green is still 100% marketing crap. Not that it doesn’t serve a useful purpose- we all worked for a paycheck to put a roof over our heads and I don’t think it was “wrong” to do so.
I like the term “blue” (thanks!) and, as an engineer(kinda) I like to think of the glass as twice as big as necessary, safety factor 2. :)
So basically hydrogen production is still a fraudulent act of greenwashing and it is actually unknow when it will become an efficient and non fraudulent process that is economically viable? Just as well there is no provable correlation between atmospheric CO2 levels and human activity. Go and look at the Keeling Curve data if you don’t believe me, it continues to rise, but there was no change in that due to the economic downturn due to government responses to covid. The signal needed to demonstrate the correlation just isn’t there. It is a genuine, hypothesis destroying, anomaly.
Using renewable energy for electrolysis of water seems to me to be the best way to produce hydrogen.
I’m in Lincoln nebraska, there’s a business not very far away that is taking natural gas as an input and outputting carbon black and anhydrous ammonia. While it’s not 100% green scheme it does serve a very important local need and also undercuts the production of carbon black by a very dirty and wasteful process of incomplete combustion of hydrocarbons. Originally they were going to have their output as hydrogen but anhydrous ammonia is more profitable. But that could change at any time once hydrogen demand goes up. I don’t think we should demand that are hydrogen comes from the greenest sources but only something which is progress. Monolith Incorporated is progress.
I have no connection with this company but I find their business to be really admirable.
These guys? monolith-corp.com/methane-pyrolysis
Seems neat, and yeah nothing wrong with ammonia; even apart from being a feedstock it is a nice alternative to plain hydrogen in a lot of ways for energy.
The most fascinating thing here is to watch those climate deniers. They still seem to be around.
What a damage can be done by the tobacco industry’s propaganda machine fueled by the Koch brothers…
The best thing you could do to help fight anthropogenic “climate change”, which was cooked up in the 1800s, would be to off yourself or the second best would be give up your phone, internet, car, house, and electricity and go back to a hunter/gatherer exiatence. Baboon
“A real concern is that many of these technologies require either a lot of electricity, or high temperatures. As noted by Sánchez-Bastardo et al., renewable energy is not likely to provide anywhere near the amount of electricity needed for electrolysis of water, even for just industrial demand. ”
I don’t see why that should apply to this process, it seems like a non-sequitur. Renewables generally produce electricity, and electric heating can surely raise the temperature of any reaction vessel to 1000C, 1500C, 2000C or whatever is required.
Besides this, we have solar arrays focusing the sun on (currently) molten salt. This application would seem to be a better use of the heat produced.
Yes, it all only works during the day, but so what? As long as we don’t cheat (see above) and oxidise the carbon later, but bury it or make it into something useful such as carbon fibre or nanotubes that keep it out of the atmosphere, it’s a win.
It makes no difference to me how it gets done. A mere two decades ago I understood that perhaps the simplest approach is a small yet sufficiently safe nuclear reactor dedicated to the hydrolysis of sea water which can be placed anywhere near a source of water.
CO2 levels in the atmosphere are dangerously low. We need more than the current levels. about 1200 ppm would be nice. Plantgrowth and thus animal growth would be enormous.
All life on earth dies at 280-ish ppm. Today we are at 440 ppm. Very close to death very close.
CO2 has none or negligent effect on earth’ temperature. It’ s effect is logarithmic and not linear so 1200 ppm is no problem at all if you are worried about temp.
Humans are a subtropical species: deaths from cold are 10 times those as death from heat.
The climate doomsday cult has it all backwards, as is usually the case with liberals.
huls’ comment reads like they’ve been stuck in a small office space at 1200 ppm for too long, which impacts human cognitive abilities ( http://dx.doi.org/10.1289/ehp.1104789 ).
>All life on earth dies at 280-ish ppm
Yeah right, that’s why we had 280 ppm during interglacial periods and ~200 ppm during ice ages. Fwiw, the limit for plant life seems to be around 50 – 100 ppm (https://www.nature.com/articles/193587a0)
Now open the bloody window.
Upper limit on a submarine is 8000 (yes eight thousand) ppm CO2. Those sailors are doing a cracking job there.
https://pubmed.ncbi.nlm.nih.gov/30511437/
Your exhaled breath is 40000 (yes forty thousand) ppm CO2. You are still capable of entering a reply. Full of disinformation but at least you got the spelling right.
C3 plants lowest limit is 150ppm, But there is no agriculture or animal sustaining growth below 280-ish.
https://notrickszone.com/2013/05/17/atmospheric-co2-concentrations-at-400-ppm-are-still-dangerously-low-for-life-on-earth/
Trying to take you seriously, but its hard…
You bump the energy in atmosphere just enough to put 0.1 Celsius on the average temps you are adding huge amounts of energy (that will never be evenly distributed) and so asking for more trouble with weather patterns, dumping CO2 up to that sort of level is going to be catastrophic just from the effect it will have on the weather… An effect its quite clear to see is starting already.
Then you have to consider the time it takes for species to migrate and/or evolve – everything living right now still expects around the pre-industrial level of carbon with pre-industrial temperatures, and as so many plants and animals are even slower breeding than humans there is no way for them to adapt that fast – so actually you would get mass extinction!
‘Deaths from Cold Vs Deaths from heat really is not a great measure either as the relative wealth and so ability to do something about it being too hot or too cold matters hugely, almost nobody lives in the goldilocks zone where you never needed for shelter and more poor folks die than wealthy is kinda a default setting for humanity – So mostly what you end up measuring by that metric alone.
You also have to realise that oh so many plants couldn’t grow any faster with more CO2 anyway – CO2 wasn’t their big growth limiting factor. Take it away significantly and you can certainly slow their growth but adding more doesn’t do anything at all as they already had more than they could use…
And as helge says high CO2 really isn’t good for cognition, and that effect starts way way under what you want to subject us all too…
The climate cult posits that we should have the most intense weather patterns ever today. This because of the CO2 concentration in the atmosphere which somehow influences the temperature of the atmosphere.
There is no sign of this at all. All extreme weather phenomena were at their lowest since 150 years.
Record lows of hurricanes in every category
Record lows of tropical storms everywhere
Etc.
When the facts change, your opinion should change. If not you’re just adhering to a religion.
For the other facts, see my reply above.
Lets not forget the most efficient method, that of utilizing the various microorganisms, anaerobes, aerobes, archaea.
Don’t do nuclear. Just use concentrating solar power. A simple fresnel lens can accomplish the needed temps.
When will the technological hopium end? Most everyone here is blind. There’s no technological solution to problems brought by technology. There’s no solution (except maybe divine intervention) at all. When will people stop believing in The Omnipotent Almighty We? Humanity cannot continue in its current form any more, and it has no choice any more.
I was reluctant to do it but I ate that banana.