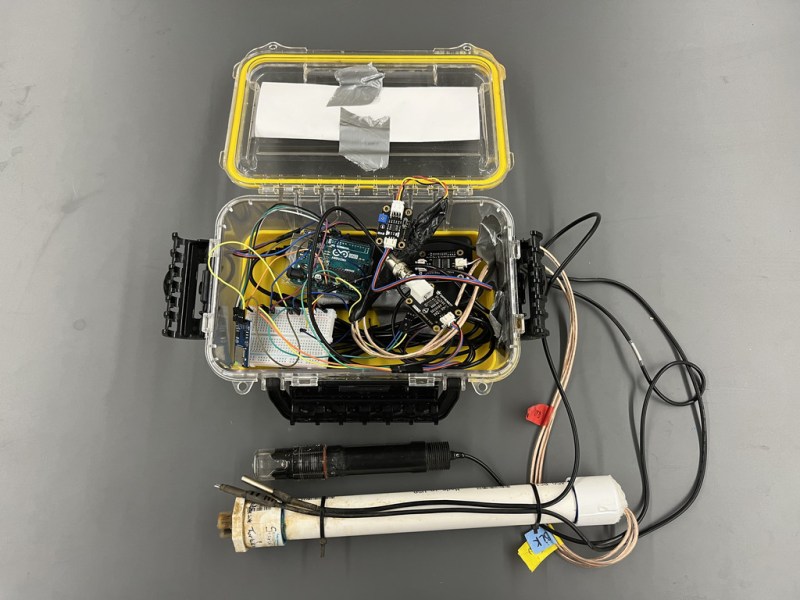
While it can be straightforward to distill water to high purity, this is rarely the best method for producing water for useful purposes. Even drinking water typically needs certain minerals in it, plants may need a certain pH, and wastewater systems have a whole host of other qualities that need to be measured. Measuring water quality is a surprisingly complex endeavor as a result and often involves a wide array of sensors, much like this water quality meter from [RowlesGroupResearch].
The water quality meters that they are putting to use are typically set up in remote locations, without power, and are targeting natural bodies of water and also wastewater treatment plants. Temperature and pH are simple enough to measure and grasp, but this device also includes sensors for total dissolved solids (TDS) and turbidity which are both methods for measuring various amounts and types of particles suspended in the water. The build is based around an Arduino so that it is easy for others to replicate, and is housed in a waterproof box with a large battery, and includes data logging to an SD card in order to make it easy to deploy in remote, outdoor settings and to gather the data at a later time.
The build log for this device also goes into detail about all of the steps needed to set this up from scratch, as well as a comprehensive bill of materials. This could be useful in plenty of professional settings such as community wastewater treatment facilities but also in situations where it’s believed that industrial activity may be impacting a natural body of water. For a water quality meter more focused on drinking water, though, we’d recommend this build that is trained on its own neural network.















“Even drinking water typically needs certain minerals in it” Really? Why?
When my wife (then fiancee) & a friend figured out simply putting a glass of water in my hand would fix a lot of problems, and my doctor’s response was “drink water” (without wondering what was being diluted & flushed away), I discovered that large quantities of tap water upset my stomach. At the time, the alternative was “spring” water (same stomach problems) or distilled, either one purchased at a grocery store in 5 (US) gallon containers.
The distilled got increasingly expensive, and I found a local vendor where you could fill your own bottles with reverse osmosis filtered water for half the price of grocery store distilled. The vendor moved east, I moved west, I found a new vendor. But, the water from the new vendor’s dispenser had a strange taste to it.
I started purchasing 4 litre bottles of distilled at the local grocery store (CA$0.97).
I have not noticed any health problems from drinking distilled, either 35 years ago, or over the last 4.
Well, distilled water usually has the effect of leaching minerals out of you (salt and so on) which is not a good idea in general.
In that sense drinking water does in fact typically need certain minerals in it.
Sure you’re not an alien or something?
Reverse osmosis does not remove all minerals from water. I think youre confusing terms.
Reverse osmosis removes 99.9% of minerals from water.
(https://www.iwapublishing.com/news/reverse-osmosis-and-removal-minerals-drinking-water)
This makes it corrosive, as it leaches minerals out of pipes etc.
Lack of minerals in distilled or reverse osmosis water also causes the water to taste dead and flat.
I wish that there was a low cost sensor for nitrogen in water, for checking levels of ammonia, nitrite, and nitrate. That would be extremely useful for aquarium and aqua-culture. The closest I’ve seen is a diy automated sensor setup that interpreted the color for the chemical test drops.
I’ve wondered a similar question in my research work… Is realtime low-cost inorganic nitrogen monitoring possible?
Uv LEDs can facilitate long term nitrate monitoring for a few K-usd$ (search NitraLED).
Pair that or some diy equivalent, with another proxy for speciation factors, like pH or oxidative reductive potential (ORP), and maybe total inorg N mass balancing is possible.
Multi point calibration of any such method, is probably a must for accuracy and defining your measurement error…
Measuring pH is simple? Tell me how to do it?
With a pH electrode. Check out the referred article.