A recent paper (PDF) in Physical Review Letters by T. Niwase and colleagues covers a fascinating new way to both create and effectively examine isotopes by employing a cyclotron and a mass spectrograph. In the paper, they describe the process of multinucleon transfer (MNT) and analysis at the recently commissioned KEK Isotope Separation System (KISS), located at the RIKEN Nishina Center in Japan.
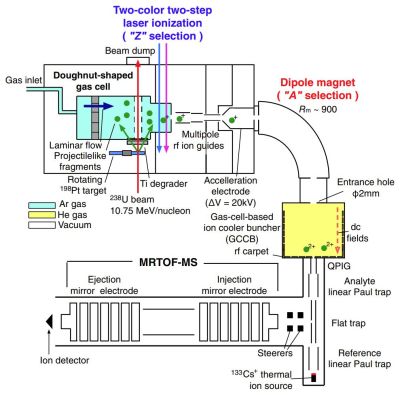
yellow-colored areas are filled with Ar and He gases, respectively. Differential pumping systems are located after the doughnut-shaped gas cell as well as before and after the GCCB. (Credit: Niwase et al., 2023)
The basic process which involves the RIKEN Ring Cyclotron, which was loaded for this particular experiment with Uranium-238 isotope. Over the course of four days, 238U particles impinged on a 198Pt target, after which the resulting projectile-like fragments (PLF) were led through the separation system (see sketch). This prepared the thus created ions to be injected into the multi-reflection time-of-flight mass spectrograph (MRTOF MS), which is a newly installed and highly refined mass spectrograph which was also recently installed at the facility.
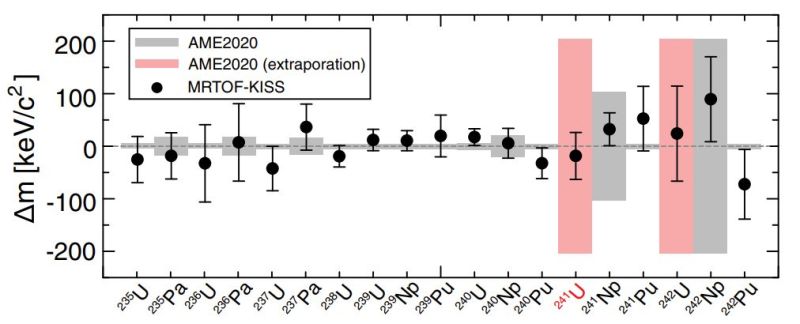
Using this method, the researchers were able to establish that during the MNT process in the cyclotron, the transfer of nucleons from the collisions had resulted in the production of 241U as well as 242U. Although the former had not previously been produced in an experimental setting, the mass of 242U had not been accurately determined. During this experiment, the two uranium as well as neptunium and other isotopes were led through the MRTOF MS instrument, allowing for the accurate measurement of the characteristics of each isotope.
The relevance of producing new artificial isotopes of uranium lies not so much in the production of these, but rather in how producing these atoms allows us to experimentally confirm theoretical predictions and extrapolations from previous data. This may one day lead us to amazing discoveries such as the famously predicted island of stability, with superheavy, stable elements with as of yet unknown properties.
Even if such astounding discoveries are not in the future for theoretical particle physics, merely having another great tool like MNT to ease the burden of experimental verification would seem to be more than worth it.















Mass Spectrometry is not the same as spectroscopy. It doesn’t involve the electromagnetic spectrum, but rather separates based on mass-charge ratio. MS purists often insist on calling the instrument a mass spectrometer to prevent confusion
Spectro – scopy –> no mater how, but by etymology you “OBSERVE” the spectrum “IMAGE”
Nowhere in the Latin there is requirement for EM spectrum.
Also great, useful hack, exactly what I had in mind for the weekend in my garage cyclotron and uranium mine.
“exactly what I had in mind for the weekend in my garage cyclotron and uranium mine.” – unless the RESTRICT Act clowns get triggered and deem you to be too great a risk…
Looks like a nice project. Might try and replicate it in my back yard.
Ah, come on. Surely you can fit it on your workbench?
Seriously HaD. Kinda drifting off the core mission here. Where’s the hack?
That’s plan B. And like someone said last week: This _IS_NOT_ Hacksaday and therefore only actually needs to publish one Hack based article per day. Anything else is a bonus.
I wondered what happened to the Radioactive Boy Scout!
I actually worked with Americium 241. It’s used in the packaging industry to inspect fill level in cans, bottles and cases of product. It is fascinating where you actually can find radioactive isotopes in the industrial complex.
just try not to lose it in the middle of the Australian desert.
Yes, it would be exceedingly difficult to find, being mostly an alpha emitter (and very few low-energy gammas, for the pedants).
The Cs-137 pellet that was lost in Australia has a wonderfully easy-to-detect 700 keV peak — easy to find just by driving by at highway speed.
That’s the neat thing about radioisotopes — they are terrifyingly (literally) easy to detect at trivial concentrations. It alarms the muggles.
I understood some of those words but my stupid friend needs an ELI5 for this article.