The magnetohydrodynamic (MHD) drive certainly sounds like something out of science fiction — using an array of magnets and electrodes, this high-tech propulsion technology promises to silently propel a craft through the water without any moving parts. As long as you can provide it with a constant supply of electricity, anyway.
Of course, as is often the case, the devil is in the details. Even with the obvious scientific and military applications of such a propulsion unit, scaling MHD technology up has proven difficult. But as [Jay Bowles] of Plasma Channel shows in his latest video, that doesn’t mean you can’t experiment with the concept at home. Even better, getting verifiable results is much easier than you’d think.
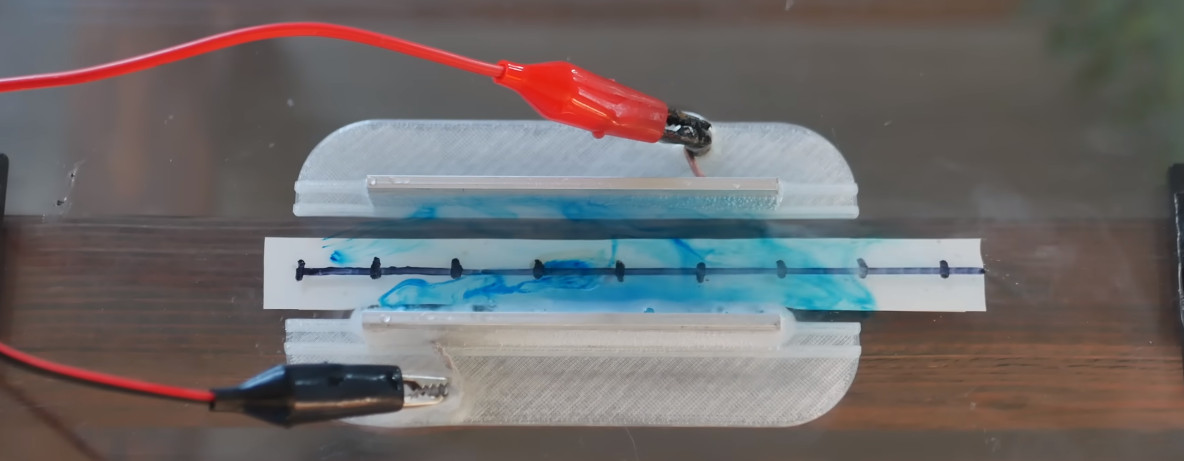
While [Jay] usually won’t even get out of bed for less than a dozen kilovolts, a standard bench supply is all you need to power your very own MHD. He ran his experiential drive at a maximum of 25 VDC/9 A, but he was getting results with just 5 VDC/1.5 A. Beyond that, it’s just a matter of cutting some electrodes out of metal stock and placing them on either side of the most powerful magnets you can get your hands on. Add in a 3D-printed structure and some pieces of acrylic, and you’re halfway to a bathtub rendition of The Hunt for Red October.
In the video, [Jay] progresses through several experiments that test variables such as the electrode spacing, magnetic field strength, and applied voltage — noting how each change impacts the velocity of water passing through his test rig. The results indicate that the MHD, at least on such a small scale, is remarkably forgiving regarding design specifics. Line your electrodes up with the magnets, flip on the power, and it seems like something will happen pretty much no matter what. If you want the best performance, the formula seems straightforward: more power + bigger magnets = higher velocity.

The final prototype thruster has a mass of 35 grams and produces approximately 75 grams of thrust while consuming 225 watts. That’s pretty impressive for a first attempt, especially for something mainly built from hardware store components.
It seems like the biggest problem is that the brass electrodes end up looking pretty rough after a relatively short time. We’re pretty sure we even saw metal flakes flying out of the back of the unit while it was operating a few times. As such, it’s unclear how long the unit could provide thrust before electrolysis really starts to take its toll. But hey, that’s what version two is for, right?
[Jay] says he was inspired to look into the magnetohydrodynamic drive after building a high-voltage catamaran, which is itself a spin-off of his ongoing efforts to build an ion-powered aircraft.
















One sink only please…
Remember Benny, some things in here don’t react well to bullets.
To increase thrust/speed reduce the out flow, widen the intake. And at the out flow create a venturi affect at the exit nozzle. The fluid should be entirely entraped on four sides allowing only intake and exhaust to be open
You never say sink when discussing boats! It’s called a basin.
Ok… thanks for the factoid.
Good thing that we’re only talking about magnetohydrodynamic drives and kitchen sinks here.
*basins
When the lavatory becomes a laboratory…
Too many rings to be safe around electricity and RF.
What would be the safe number?
One.
Zero. When I went out on the job helping install SCADA systems, the gold wedding ring went in the pocket. Of course I wasn’t working around just 5V…..
Yup, there’s a guy on YouTube who repairs e-bike batteries and he’s got rings on every finger, he’s basically begging to have a finger or two cauterised off and an exothermic battery pack fire
This is why my wedding ring is titanium
Mine is plastic
Suggest this method if this ever happens to you. Photos of actual event at link:
Titanium Rings Tough To Crack In Emergencies
https://www.npr.org/sections/health-shots/2015/08/13/432086018/titanium-rings-tough-to-crack-in-emergencies
Now that finger was painful and swollen. The ring needed to come off, since restricted blood flow can lead to tissue death in the finger, which is about as fun as it sounds.
Ordinarily, this wouldn’t be much of a problem.
A normal ring cutter won’t necessarily work, says Dr. Andrej Salibi, a plastic surgeon at Sheffield Teaching Hospitals in the U.K. and co-author of the letter.
In this case, the ring cutter failed, and the fire department came in with its own specialized cutting gear. The ring wouldn’t budge. The patient was admitted to the hospital and spent the night with his hand elevated. The next morning one of the physicians suggested they try something else, namely bolt cutters, which are often on hand in hospitals.
It worked! But “the other problem is that once you cut it, you have to take it off,” says Salibi. And that takes a lot of force. So using some large, heavy-duty paperclips, the two physicians pulled the ring apart. The man’s finger was fine. The doctors say bolt cutters are preferable to dental saws or diamond-tipped saws, which aren’t likely to be lying around the hospital and require more manpower.
This device should be standard equipment in ER places.
https://www.ringrescue.com/compression-device
Almost afraid to ask why it’s so common. Someone lock themselves out of the supply cabinet again?
so, we pollute the water with metal instead of noise. I mean we are dissolving metal to accomplish this right?
Gold plating should help. It’s the lorenz force that pushes the water.
If Lorentz force, why do the electrodes need to be exposed in the first place? Casting them in a thin potting material shouldn’t effect efficiency that much.
We still need to electrify the water. I have forgotten all my high school chemistry after two decades, but would _all_ electrodes dissolve? Or could one have an electrode made of a non-metallic conductor (say graphite) or even a less reactive metal (like gold as suggested by Ron)?
There’s trace amounts of all sorts of metals dissolved in ocean water.
Dissolution of metal isn’t a required part of the process that produces the thrust. He is getting hydrolysis as a side reaction, which is causing *oxidation* of the metal, but that’s *adding* material to the electrodes. I believe no metal is being dissolved here.
I dunno about brass right off hand, but I know that with e.g. pure copper, the oxide has different enough inter-atomic spacing that the oxide easily flakes off. So while technically, yes, the brass isn’t going into solution, it is for sure coming off of the electrode. Its small comfort to know that the material coming off of the electrode is not dissolving.
The electrode pair oxidizes at one electrode, and reduces at the other.
graphite electrodes maybe ?
How about “some platinum. A small block would be sufficient, five or six pounds.”?
in this zinc plated, vacuum tube culture ?
Hmmm. Somehow I’m thinking adding carbon or carbon steel. Activated carbon can induce magnetic fields by changing proton spin of water, however… It may or may not effect thrust and only create hydrogens.
“Activated carbon can induce magnetic fields by changing proton spin of water,”
What kind of Woo or (witchcraft) is this? Got a reference?
So it can’t help me with the dishes?
Thank you this comment is what I was looking for
If only dishes can be charged to repel any non-dish particles
Imagine a wrench you grab a dirty dish and electro wrench it and in a zap 3 day old noodles are my new kitchen wallpaper
Mike
Table salt has metal in it FYI. It wouldn’t be a salt otherwise. :D
The oceans are LOADED with metal … literally. Besides you NEED that metal to stay alive so … there you have it. :D
love tech ingredients?
https://www.youtube.com/watch?v=LS3GQk9ETRU
Although he doesn’t start writing physics equations and giving gory details on how to calculate force etc. This person gives a good enough amount of detail most people can find the discussion interesting.
Yes, but that is mostly chemically bound into a non-toxic molecule, sodium chloride. I don’t recommend eating elemental sodium, or any of the other metals present in the ocean for that matter.
Also, the copper which makes up a large portion of the alloy known as brass is very toxic to marine life.
The sodium in solution in the sea is not “bound into” anything. There are sodium ions, and there are chlorine ions. They are not connected. That is what “dissolved” means, when you’re talking about ionic compounds.
Sodium chloride non-toxic? Only at low enough levels. Just like most non-heavy metals. (And even heavy metals have to accumulate sufficiently to cause effects.) Like most things, dosage is important. Even pure water can be toxic in sufficient quantities.
I don’t recommend drinking seawater, either. You aren’t evolved to eject the excess sodium and chloride without dumping more water than you consumed.
Thank God I’m not a fish
Gas, grass or brass is what I tell vegans
Water’s diamagnetic IIRC, wonder if this could be done with no electrolysis and huge magnetic fields (useless for a stealth drive obvs)
This was my first thought when I saw those hydrogen bubbles – that electrolysis is wasting energy!
Re: Halbach arrays – that should increase specific field strength, but I’m not sure if the field direction would produce more thrust.
To avoid losing so much energy to electrolysis and reduce electrode corrosion, it would be interesting to try and use electromagnets and drive both the magnets and current with AC as in this 2000 paper: http://dx.doi.org/10.1016/S0925-4005(00)00355-5
The bubble threshold (voltage at which electrolysis begins) increases with electrode drive frequency while the field strength decreases (presumably as reactance also increases) with magnet drive frequency. It doesn’t look like they tested driving the two circuits with different frequencies, only varying the phase.
I wonder if using litz wire for the electromagnets would allow the frequency to be pushed high enough to eliminate most of the electrolysis while still getting good thrust.
Of course, if you can get higher specific field from electromagnets than fixed magnets then that’s one reason to use them regardless – you could keep the voltage lower and get less electrolysis. The energy cost is higher due to Joule heating, but at least you can get water cooling for free if you design it right!
i also wanoder if you could use AC electromagnets and insulated electrode plates and drive them with higher valtage AC and use only the so built capacitance displacment current.
‘Sneaky’ unless you live sometime after the 1930s when MADs were invented for submarine hunting. In which case a MHD is yelling to every magnetometer even vaguely nearby “HEY EVERYONE! I’M BEING SNEAKY!”.
How does the magnetic flux created by an MHD compare to various electrical devices inside any submarine?
Also, from what I understood from the wiki article on MAD (https://en.wikipedia.org/wiki/Magnetic_anomaly_detector), MAD has a very short range. I imagine MHD would be advantageous when trying to evade passive sonar (https://en.wikipedia.org/wiki/Sonar#Passive_sonar) until you are within MHD range, at which point just the metal structure of the sub would be sufficient to trigger the MAD anyway.
Really neat work! Minor physics nitpick, it’s not the voltage that makes things happen, it’s the current (or amperage to avoid possible ambiguity). More salt would let you reduce the 25 volt drive needed to maintain the 9 amp current and you would get the same water flow rate at less power. You can drive mercury or other molten metal very efficiently with very small voltages for the same current. This is (essentially) the difference between building a motor using steel wire windings versus using copper wire windings.
I would imagine he could improve the efficiency here either by using Hallbach arrays or by using a yoke around the outside. Might not improve it enough to offset the weight gain though.
Although I called it a silent sea water engine, the subject of my 1970 science fair project was on magnetohydrodynamic Drive. I won several awards at the district and state level for my project.
Yamato-1 on display in Kobe, Japan first working prototype of magnetohydrodynamic (MHD) drive. Shows ship, drive side view and exhast end view.
https://en.wikipedia.org/wiki/Yamato_1
Thunderbird2!
The circle of discovery,,in the 60’s maybe 70’s Popular Science had an article describing a stationary electric unit to move saltwater, no though about a submarine. Earworms move forward in time.
Perhaps it was the January, 1966, ( page 112 ) article titled “Silent Sea Engine for Nuclear subs” by James G. Busse? Includes directions on how to build a working model.
Because otherwise current would not flow through the water and the water would not be pushed out the back by the magnetic field.
Replace the permanent magnets with an electromagnet or perhaps even use superconductors. I wonder what the limit to this is. No doubt if you move the water too fast the pressure difference will start to allow it to boil.
This is a thruster, we expect a thrust measurement (N), why did he not strap the thing to a load cell to get one!
225w for 0.22 lbs of thrust. Great, just need to provide 40-50kw of power for an equivalent average boat motor.
In the 70’s there was research on a MHD generator. Finely ground coal or natural gas was combusted and the hot gasses streamed through the MHD chamber generating electricity, then the hot gasses were sent through a steam generator creating more power. The efficiency predictions were in the neighborhood of 70% with lower emissions. In the case of coal the ash was captured and disposed of. The main problem was erosion of the main channel but a ceramic coating was being experimented with.
Many years ago, 1970’s or 1980’s, I recall an article about a research submarine with an MHD drive. It had no room for people, was just for testing the drive. It was all sealed so the magnetic coils and other equipment weren’t in contact with the sea water. It created a magnetic field in the water and an opposing field in the sub to push against one another. Shove water aft, Newton’s 3rd Law of motion makes the sub go forward, slowly.
Hi guys, great project. You used salt water for this experiment, and I was wondering how much chlorine gas did you produce? Water being H2O and salt being NaCl…
None. Electrolysis of water (even salt water) does not release sodium, or chlorine, just Hydrogen and Oxygen.
I have an eerie feeling everyone here isntisn’t talking about chemistry