If you’ve got an interest in technology, it’s inevitable that your feed will feature a constant supply of stories with titles in the vein of “New battery breakthrough offers unlimited life and capacity!”. If we had a pound, dollar, or Euro for each one, we’d be millionaires by now. But while the real science behind the breathless headlines will undoubtedly have provided incremental battery improvements, we’re still waiting for the unlimited battery.
It’s not to say that they don’t conceal some interesting stories though, and there’s an announcement from Australia proving this point admirably. Scientists at ECU in Perth have created a new cathode compound for rechargeable zinc-air batteries, which it is hoped will make them much safer and cheaper competitors for lithium-ion cells.
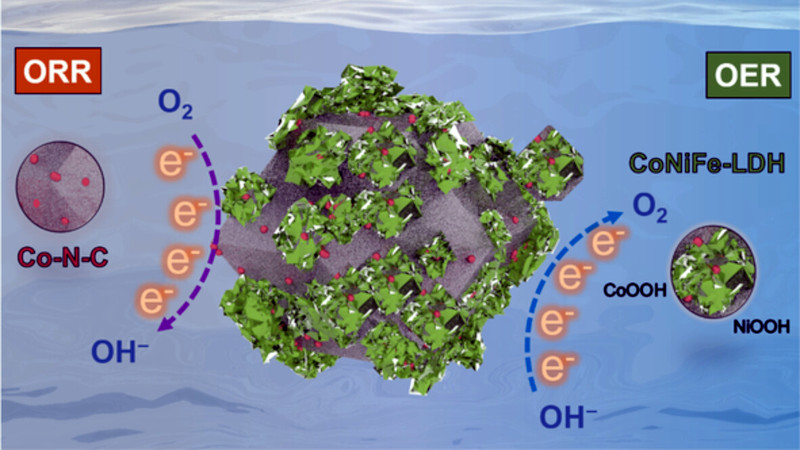
Most of us think of zinc-air batteries as the tiny cells you’d put in a camera or a hearing aid, but these conceal a chemistry with significant potential that is held back by the difficulty of creating a reliable cathode. In these batteries the cathode is a porous support in which a reaction between zinc powder wet paste and oxygen in the air occurs, turning zinc into zinc oxide and releasing electrons which can be harvested as electricity. They have a very high power density, but previous cathode materials have quickly degraded performance when presented with significant load.
The new cathode support is a nano-composite material containing cobalt, nickel, and iron, and is claimed to offer much better performance without the degradation. Whether or not it can be mass-produced remains to be seen, but as a possible alternative to lithium-ion in portable and transport applications it’s of great interest.
















If they can realize the double- or triple- the energy density of lithum batteries, that’s great. It will enable lighter, less resource-intensive and lower carbon-footprint EVs.
But they still have the issue of requiring cobalt and nickel — no not addressing the most serious limitation of lithium ion batteries.
Then everyone can drive around with the equivalent of a half tonne of thermite under their car.
Fascinating! I would love to see the math.
4 MJ/kg * 500 kg = 2 GJ = 556 kWh
It would surprise me to see passenger vehicles with more than 100 kwh or so. Maybe 150 for the bigger / heavier SUVs and cosplay trucks.
At 100kwh you can probably drive 5 hours between charges. At that point, aren’t there better uses for that volume and mass? I doubt that many buyers are going to pay extra just so that they can plug it in once a week instead of plugging it in overnight.
> just so that they can plug it in once a week instead
Thing is, you don’t want to be living “hand to mouth” where you don’t have any spare range. Being able to charge just once a week means you can pick the day of the week when electricity is cheap because of wind power etc.instead of having to charge every single night just to be barely able to get through the next day.
Also, you have to account for bad weather and winter where your range will halve, and then battery aging where you’ll lose up to half of your range anyways, so you’ll need at least enough capacity to last you 4-5 days when new so the same battery can still serve you for 1 day when old.
Zinc is also prone to dendrite growth that damaged the cell. Need to overcome this anamoly.
If it were a Tesla, it’d be marketed as a self-destruct feature.
But would Ethan Phillips drive one?
…equivalent of a half tonne of thermite under their car.
As opposed to the equivalent of half tonne of TNT in the fuel tank of a similar-weight fossil fuel vehicle…
Except gas is pretty darn stable. And you can put the fires out :)
Metal-air batteries are stable in the same sense as gasoline is: limited access to the oxidizer.
If it was a regular battery with equivalent energy density, you wouldn’t want to be anywhere near it in a crash.
You mean, like having a small tank of gasoline?
Thermite has its own oxydizer. Zinc-air batteries use oxygen from the air.
Another challenge for metal-air batteries is the need for clean air. Dust is obvious, but things like sulfur dioxide and other volatile compounds in polluted air will poison the cells and degrade performance quickly.
Oh my goodness. Industrial incentive to clean the air. That’s perfect.
Even trees put out volatile organic compounds that would poison a metal-air battery cell over time.
And compressors to force it through the porous electrodes. High rate xx-air batteries and fuel cells all suffer from a lot of supporting mechanical/gas hardware vis-a-vis batteries. And then they still have all the batteries and support gear for peak power.
(Micro gas turbines also seem to be like this – a turbine genset seems super simple, small, and cheap, until you add the compressors, filters etc, etc)
At least the turbine can burn most of the stuff, or just pass it through with little ill effect. Chemical cells don’t like any sort of solid particles, and they react with carbon monoxide, sulfur, etc. which degrades the catalyst, so the problem of filtering the intake is much worse.
Are the fossil fuel companies still buying up all the battery patents and sitting on them? Because that’s part of the reason why we hear about all those discoveries, but never see them put to use. Patents are such a boost to innovation!
More like it’s hard going from lab to economical.
They fix one thing and break another – hard to get everything going right at the same time.
This 👆
Quote: “If we had a pound, dollar, or Euro for each one, we’d be millionaires by now. ”
That is why i “nuff said.”
Let us wait until it hits the shelves, is build into things and sold. Everything else is vaporware.
It has to be COTS before I look at it.
Commercial Off The Shelf
I also call this the Aisle 47 question. When someone tells me about a new technology, I want to be able to find it on Aisle 47 at my local Wal-Mart. Not that many ‘new technologies’ get there.
Especially since there is no such aisle – Walmart uses a letter and number combination for aisle identification.
Found the walmart employee.
I backed a cloud generator on Kickstarter. Turned out to be vapourware.
The feasibility of zinc-air accumulators is known since the 1970s, a key element being gas diffusion electrodes developed for fuel cells. Currently many universities and research agencies are revisiting this “old” technology, with “breakthroughs” announced every month or so.
IMHO we won’t see Zn-air cells in vehicles in the next decades, but they could be an alternative to Li cells in portable devices.
Backup for the grid with less chance of spontaneous combustion.
https://www.wired.com/story/big-grid-batteries-are-booming-so-are-fears-fire/
Could be perfect for eBikes.
An interesting issue with metal-air batteries is that they get heavier as they discharge due to the weight of the reacted oxygen. Not exactly optimal for a vehicle battery. You’d have a positive feedback loop where a heavier vehicle has less available charge to move itself.
Zinc has an atomic mass slightly over 65 while oxygen’s is 16. So even if a battery was 100% zinc at full charge and 100% zinc oxide when spend that would only be a 25% mass increase for the battery. And the battery is far from the only heavy part of a car. Not nothing but not a deal breaker.
It’s worth noting that rechargeable AA nickel-zinc cells are already available on Amazon, and I’m pretty happy with the performance. One advantage of NiZn cells is the cell voltage is 1.6V, so no issues with some devices being unable to use them, and they also don’t heat up while charging. They’re not as dense as comparable lithium rechargeable AA cells (2.5WH vs 3.5WH), though it will be interesting to see how much they can be developed.
*Sigh* I was excited about some new tech supplanting lithium tech because of the deep ethical problems with cobalt-based cathodes… read the ingredients list and got sad. Basically they’ve made zinc as unjust as lithium.