The process of creating new battery chemistries that work better than existing types is a slow and arduous one. Not only does it know more failures than successes, it’s rare that a once successful type gets completely phased out, which is why today we’re using lead-acid, NiMH, alkaline, lithium, zinc-air, lithium-ion and a host of other battery types alongside each other. For one of the up-and-coming types in the form of sodium (Na)-based batteries the same struggles are true as it attempts to hit the right balance between anode, cathode and electrolyte properties. A pragmatic solution here involves Prussian Blue for the cathode and hard carbon for the anode, as is the case with Swedish Northvolt’s newly announced sodium-ion battery (SIB) which is sampling next year.
The story of SIBs goes back well over a decade, with a recent review article by Poonam Yadav and colleagues in Oxford Open Materials Science providing a good overview of the many types of anodes, cathodes and electrolytes which have been attempted and the results. One of the issues that prevents an SIB from directly using the carbon-based anodes employed with today’s lithium-ion batteries (LIB) is its much larger ionic radius that prevents intercalation without altering the carbon material to accept Na+ ions.
This is essentially where the hard carbon (HC) anode used by a number of SIB-producing companies comes into play, which has a far looser structure that does accept these ions and thus can be used with SIBs. The remaining challenges lie then with the electrolyte – which is where an organic form is the most successful – and the material for the sodium-containing cathode.
Although oxide forms and even sodium vanadium fluorophosphate (NVPF) are also being used, Prussian Blue analogs (PBAs) are attractive for being very low-cost and effective as cathode material once processed. An efficient way to process PB into fully sodiated and reduced Prussian White was demonstrated a few years ago, followed by successive studies backing up this assessment.
Although SIBs are seeing limited commercial use at this point, signs are that if it can be commercialized for the consumer market, it would have similar capacity as current LIBs, albeit with the potential to be cheaper, more durable and easier to recycle.
















Sodium-ion batteries, while not able to solve all our problems, will still solve a large number of them. You’re already able to get your hands on some in smallish (18650) form factors (although they are still rather expensive), so naturally, I’d like to tinker with them. However, I’m unable to find sodium-ion charger ICs. Can anyone give me pointers or are they just not available yet?
I used a CCCV charger that I custom built. I submitted it as part of the Op-Amp contest earlier this year under the same username. I have been able to do capacity tests after charging at 1C (to 0.1C cutoff) at different voltage setpoints (3.0v to 4.3v). You can absolutely use a 4.2v setpoint charger for standard lithium chemistries, but I cannot say how reduced the cycle life becomes. On the flip side I was able to get 1600mAh at down to 1.5v discharge at 1C from a 1300mAh cell when it was charged to 4.2v.
Hopefully tonight I will have enough written down to add another project on the hackaday.io part of the site.
I just plugged mine into the mains. They blew the eff up so perhaps don’t do that.
What about sodium iron phosphate?
Or potassium ion or potassium iron phosphate
Alkalai group have the same reactions what works for hydrogen or lithium should work all the way down to cesium and Francium
It’s scary enough imagining a random member of the public being able to unroll a lithium battery and start playing with a sheet of explosive metal, I dare not contemplate sodium let alone moving dowm to cesium. Although I guess the next generation of Elon’s cars would be news entertainment.
Random member of the public being able to buy 1-1000 liter of explosive fluid at gas station…
There was that one youtube video where a guy built a “shotgun” out of parts found at an airport Tax Free shop. One of the parts was lithium batteries.
Let’s ban airports.
Almost absolutely sure these batteries don’t contain the metallic form of these metals, they use salts that contain the metal ions (hence the lithium ion part of the battery name) that are much less … energetic when compared to a chunk of lithium or sodium metal (though they are still flammable when they reach their flash point of course). The anode and cathode themselves iirc are copper and aluminum.
Is it Prussian Blue or elemental sodium?
Jehu has some to play with.
https://jag35.com/collections/new-arrivals/products/50-000-cycle-battery-12v-sodium-ion-5-6ah-25c-140a
The most important thing about using sodium is that it’s scalable to global proportions. We can easily imagine a global-scale grid using sodium-based batteries to store energy and balance solar power, not just on a daily basis but also anual.
Trying to REALISTICALLY imagine the same thing with lithium-based batteries is hard. It’s too rare, and what’s available is constrained to a few localities (such as China). Even taking ALL of the lithium dissolved in the ocean wouldn’t be enough, by several orders of magnitude IIRC.
It looks like China only produces about 14% of the world’s lithium: https://www.visualcapitalist.com/visualizing-25-years-of-lithium-production-by-country/ I think you should recheck your numbers on viability too, as lithium is more prevalent in the earth’s crust than nitrogen, copper or zinc, for example. (But I agree that sodium ion has lots of potential)
True, but I believe there is more sodium in the ocean than we can mine lithium, so it is not nearly as scarce a resource. I can’t speak to the viability/cost of capturing sodium from the ocean, but considering the salinity of the ocean is 3-4%, there’s likely way more to find there than mining lithium by several orders of magnitude.
Lithium may be “more prevalent in the earth’s crust than nitrogen”, but barely so [https://en.wikipedia.org/wiki/File:Elemental_abundances.svg from https://en.wikipedia.org/wiki/Abundance_of_elements_in_Earth%27s_crust%5D. Anyway, it isn’t relative abundance that matters, but availability based on ores with high concentrations.
The BEST future I can forsee for lithium-based battery technology is early growth, followed by frequent massive price shocks and finally abandonment of the technology that uses it, LONG before it has scaled to global levels.
Probably to be replaced by technology based on sodium, or iron, or some other massively available element.
Huge lithium deposits have recently been found in Nevada and in India.
Also, geothermal power plant projects are excellent and more environmentally friendly sources of lithium and other needed minerals. That includes enhanced geothermal, not just where geysers and hot springs are. See the Salton Sea geothermal project in California for an example. And batteries can be nearly 100% recycled.
You can recycle Li with acid/oxidative leaching, yeah but you end up with same environment issues waste water problems, so prefer sodium any day
I’m well aware of recent lithium finds [https://twitter.com/RamblingAK/status/1701235381583163845]. But AFAIK “geothermal power plant projects” aren’t likely to be a source of ANY elements in useful amounts. And batteries can only be recycled in the amounts that already exist. To expand grid balancing of “intermittent” energy such as solar to global proporations, it would be necessary to create a LOT of batteries.
One downside of sodium ion is the discharge voltage, which has a steep slope. (LFP is the polar opposite of that, with a voltage that’s stable at 3.2V for most of the cycle.) Powering directly from the battery will not be feasible in most applications; power conversion is needed. That can matter in radio applications where you want to minimize noise, because noise directly affects the sensitivity of the receiver.
How does the voltage slope compare to lead acid?
Lead-acid goes from about 1.8 volts to 2.2 volts per cell, while the sodium battery appears to go from 1 to 2 Volts, so one changes about 20% and the other about 50% over a full charge.
But then, we don’t know how linear that is, and temperature matters a lot.
Upside could be easier and more accurate state of charge/health measurement
No battery SoC measured from voltage alone is accurate without counting coulombs, and temperature, and internal resistance, and…
For lithium batteries that’s pretty much because the discharge curve is so relatively flat, making the discharge voltage alone an unreliable indicator of remaining charge. But if the discharge curve of sodium ion batteries has a steep slope then it’d possibly be a better indicator (maybe “accurate” was the wrong term to use, and “more accurate for practical consumer purposes” would have been better).
I recall reading years ago that chlorine bleach is cheap because chlorine is thecwaste product from sodium manufacturing. I’m thinking the ideal battery chemistry would also use the chlorine, to avoid filling whatever with vast amounts of chlorine gas.
Don’t worry, they use the chlorine to make PVC, which is why we live in houses covered in it and drink from pipes made of it. I’m sure they’ll find more ways to sell us our own trash.
Erm, where the hell does anybody use PVC pipes for drinking water? Not in the USand Europe for sure.
PVC pipes for fresh water are common in the U.S.
Astounding.
Apologies to Anonymous for thinking he was crazy when he said that.
Addendum: You are probably confusing PEX with PVC right? PEX is Cross-linked polyethylene and not PVC.
PVC is also legal for water distribution in addition to PEX as long as it’s not hot water (for hot water, CPVC is permissible).
Common in older houses in the US, mostly outdoors. It’s infamous for becoming brittle with UV exposure and then bursting very easily.
Welcome to the rest of the world.
I don’t know what specific type but most water pipes are plastic now in the UK, much cheaper than copper plumbing in the house and way better than the old cast iron etc. supply pipes in the roads.
John Guest Speedfit are what your plumber would usually be turning up with, the pipe has several layers. It’s a reliable system too, and any repairs are easy.
In the UK it’s definetly PEX or similar and not PVC.
I Australia we use PVC for sewer and stormwater. If it is outside it must be painted to withstand UV exposure from the sun
Cl isn’t a waste product, it’s the commodity chemical with the largest production volume worldwide, 4.8 Mt/a in Germany alone. And if you’re wondering where all that Na goes: NaOH is #2.
AFAIK the biggest use of chlorine is in drinking water disinfection [https://www.health.state.mn.us/communities/environment/water/factsheet/chlorination.html]. Much of the world currently lacks clean, safe, drinking water, so projects to electrolyse salt (NaCl) producing chlorine and hydrogen could actually be a good way to get hydrogen from nuclear (including solar) power when it isn’t needed for the grid.
TEMPORARILY. It wouldn’t scale to global proportions (AFAIK). But for the immediate future, hydrogen for energy storage could be generated this way, while the technology for direct electrolysis of water (to H2 & O2) matures.
Since the process also produces sodium hydroxide (NaOH), which can be purified/electrolysed to sodium, it could also support sodium batteries.
IIRC (from old searching I couldn’t quickly duplicate) release of chlorine into the atmosphere in drinking water quantities would be fairly safe as it is quickly scavanged by the ocean. IDK about larger quantities. (Of course, when chlorinated drinking water is mixed with sewage or agricultural waste, it usually gets tied up oxidizing it and never makes it into the air.)
I noticed that there is renewed interest in Prussian Blue before.
And no Prussian lawyers to enforce the trademark!
Is P Blue flammable like Lithium?
It’s an oxide so no. Sodium is flammable though, it explodes on contact with water.
Iron oxide is one of the ingredients of thermite isn’t it?
Looks like you can get 18650 type and other shaped Na batteries on Alibaba. The discharge voltage isn’t very flat like Lithium. The energy density also is half or less than comparable Li Ion batteries. But they appear to be cheaper (which they should be.)
Very interesting future for Na Ion batteries.
The article failes to mention energy density. Sodium ion batteries do not have the same energy density as lithium ion batteries. That means, more sodium ion batteries are about half that of Lithium ion. It also does not touch on the longevity problem with Sodium ion batteries. The hope is prussian blue cathode and other improvements increases charging cycle and the life span cheaply. Sodium ion likely won’t supplant Lithium ion batteries, but they could be a big improvement over some roles that are traditionally suited to cheap lead acid batteries. It’s a big ‘if’ that they will hold up to the hype and overcome some frustrating limitations.
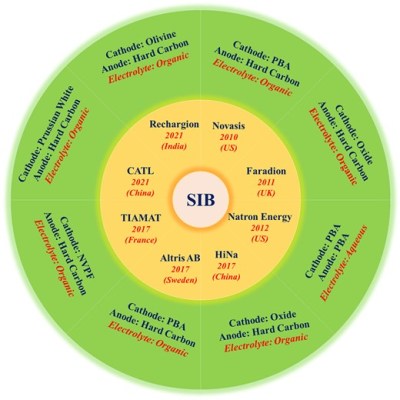
What the heck is that green circle infographic supposed to be communicating?
Sodium ion batteries may have one particular and worthwhile use as the storage batteries for the night time part of the solar farm energy capture (day time) and night time energy storage part of the cycle.
Lithium has failed badly here several times, costing millions of dollars.
Sodium ion batteries should be safer, and, though much heavier, that weight increase is of little importance because that bank of batteries remains fixed to the ground.
Great, another “breakthrough”.
Find me the battery technology that’s energy dense, safe, and doesn’t have a bunch of poor people from third world countries scrapping through the mining detrius to scrape enough toxic material together so they can feed their families and I’ll be listening.
This electric battery powered revolution we’re seeking comes at a price. All energy comes at a price. I would prefer we weigh in ALL factors before insisting on a green energy revolution.
If giving a crap about some kid in a foreign country that has to work to power some “green” technology here at home makes me a bleeding heart liberal in your eyes, I pity you.
I’m human and give a crap. Don’t like it, shove it.
Hear hear!
Yes, all energy comes at a price, and when we weigh all factors it’s pretty clear that the price is a lot higher when you use fossil fuels. Did you know that refining gasoline uses cobalt, while LFP batteries do not?
You can bribe a US congressperson for as low as $2K, so why doesn’t Apple or a group of companies bribe the leaders of the countries where they have child labor to make them outlaw it? It is probably peanuts for Apple and cheaper than that fake ‘we use second hand cobalt so that the children can’t blame us even though they still mined it haha’ fix. I mean it’s a case where nobody can be upset about bribing. and no prosecution will be done, and if so, they can afford the fine.. or make congress make an exception law.
And if a collaboration of companies including multi-trillion bucks Apple feel it’s too ‘expensive’ then partner with nations, I’m sure you can even get China to pitch in.
That problem and batteries are barely related if related at all – look at even something as ‘safe’ as clothing for instance, may not be actively toxic environment but the work practices of the sweatshop can amount to a much the same hazard. That problem is the greed of the international corporations and individuals in power in those nations so worker safety is never implemented along with the opaqueness that means people who are aware and care the problem exists still can’t actually know the working conditions that created the product they bought.
Also I’d suggest this being sodium based is potentially exactly that non toxic technology, sodium is everywhere to the point it might even be cheaper to desalinate sea water and then further process the salt to whichever form of sodium is desired right at the battery factory than ship it from a mine anywhere but on the doorstep – shipping isn’t that expensive but pure cost, where desalination the ‘waste’ products from the batteries point of view are likely still essential products for some other industry – doesn’t need to be profitable to see the fresh water and chlorine, just offset the costs sufficiently its more profitable than the mining and shipping from somewhere else.
It’s not really about saving the planet, it’s at best a money making grift marketed as “saving the planet.”
Yup. Too many things (see solar Frickin’ Roadways and the money they grifted) that we’re being shown are vaporware. They exist for only the ability to take investor money and act as if they’re finding solutions. Physics will not be denied. Energy is energy. Work is work. When all the calculations are done are any of the ideas being presented any better than what we’ve already achieved? If so, by how much? What’s the return on design investment?
A slow move to the electric powertrain future may be possible, but all these shortcuts and lab experiments that might be promising are decades away from real practical use. Our power grid is ill equipped for the electric transition. Our power generation isn’t green to a great extent. The power needed to create the Green power generation systems requires carbon power for now, and how much is actually released while building that solar panel or wind turbine? Will it ever recover and offset as much carbon as was required for making it and installing it and the infrastructure needed to put it where effective?
Gen IV nuclear is a good path forward, but shunned for ill informed reasons. The energy density makes sense and if we standardize the construction we can in a few years offset any carbon based energy needed to create a plant. But somebody who invested in solar and wind is taking your attention away from effective means of solving the carbon crisis (they insist exists).
Follow the money. Like Physics it doesn’t lie.
After doing a quick Google, most results show that a wind turbine will be carbon neutral in 4-9 months.