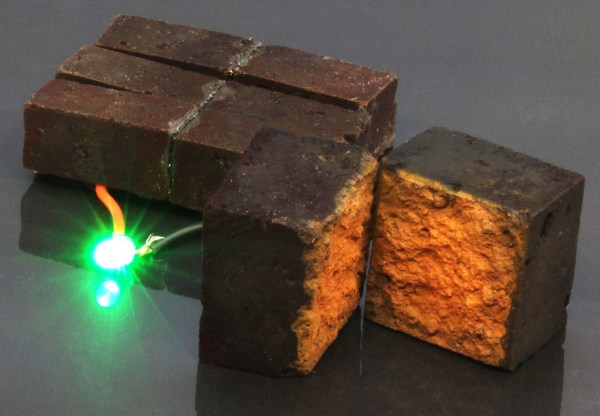
What if building an emergency battery were as easy as painting conductive plastic onto bricks, stacking them, and charging them up? Researchers at Washington University in St. Louis have done just that — they’ve created supercapacitors by modifying regular old red bricks from various big-box hardware stores.
The bricks are coated in poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT:PSS), a conductive polymer that soaks readily into the bricks’ porous surface. When the coated brick is connected to a power source such as a solar panel, the polymer soaks up ions like a sponge. PEDOT:PSS reacts with the iron oxide in the bricks, the rust that gives them their reddish-orange color. Check out the demonstration after the break — it’s a time lapse that shows three PEDOT-coated bricks powering a white LED for ten minutes.
We envision a future where a brick house could double as a battery backup when the power goes out. The researchers thought of that too, or at least had their eye on the outdoors. They waterproofed the PEDOT-coated bricks in epoxy and found they retain 90% of their capacitance and are still efficient after 10,000 charge-discharge cycles. Since this doesn’t take any special kind of brick, it seems to us that any sufficiently porous material would work as long as iron oxide is also present for the reaction. What do you think?
If you can get your hands on the stuff, PEDOT:PSS has all kinds of uses from paper-thin conductors to homebrew organic LEDs.