One of the essential problems of bio-robotics is actuators. The rotors, bearings, and electrical elements of the stepper motors and other electromechanical drives we generally turn to for robotics projects are not really happy in living systems. But building actuators the way nature does it — from muscle tissue — opens up a host of applications. That’s where this complete how-to guide on building and controlling muscle-powered machines comes in.
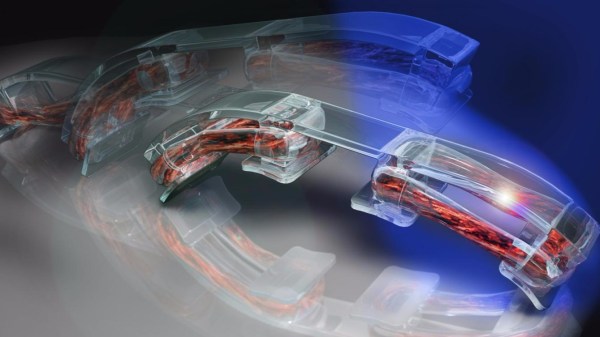
Coming out of the [Rashid Bashir] lab at the University of Illinois at Urbana-Campaign, the underlying principles are simple, which of course is the key to their power. The technique involves growing rings of muscle tissue in culture using 3D-printed hydrogel as forms. The grown muscle rings are fitted on another 3D-printed structure, this one a skeleton with stiff legs on a flexible backbone. Stretched over the legs like rubber bands, the muscle rings can be made to contract and move the little bots around.
Previous incarnations of this technique relied on cultured rat heart muscle cells, which contract rhythmically of their own accord. That yielded motion but lacked control, so for this go-around, [Bashir] et al used skeletal muscle cells genetically engineered to contract when exposed to light. Illuminating different parts of the muscle ring lets the researchers move the bio-bots anywhere they want. They can also use electric stimulation to control the bio-bots.
The method isn’t quite at the point where home lab biohackers will start churning out armies of bio-bots. But the paper is remarkably detailed in methods and materials, from the CAD files for 3D-printing the forms and bio-bot skeletons to a complete troubleshooting guide. It’s all there, and it could be a game changer for developing the robotic surgeons of the future.
Continue reading “Genetically Engineered Muscle Cells Power Tiny Bio-Robots”