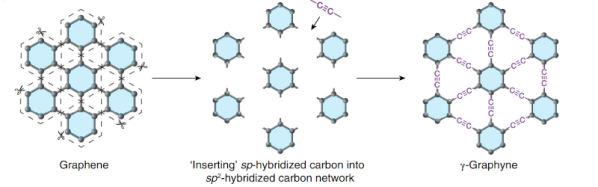
Before you jump down to the comments to chastise us for misspelling graphene, note that graphyne is similar to graphene but not the same. Like graphene, it is a two-dimensional structure of carbon. Unlike graphene, it contains double and triple bonds and does not always form hexagons. Scientists have postulated its existence for decades, but researchers at the University of Colorado Boulder have finally managed to pull it off. You can also download the paper if you want to wade through the details.
Carbon forms like fullerene and graphene are well-known and have many novel uses. Other allotropes of carbon include graphite and diamonds — certainly two things with wildly varying properties. Graphyne has conductivity similar to graphene but may also have other benefits.