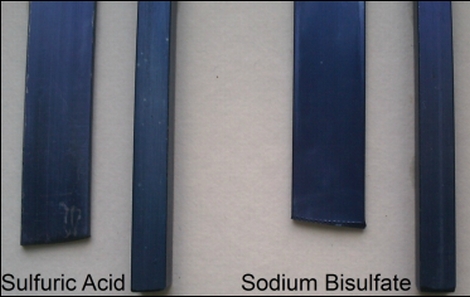
While many people have tried their hand at anodizing aluminum at home, there are plenty who would just as soon leave it up to the professionals due to the highly concentrated sulfuric acid required for the process. [Ken] started thinking about the process and wondered if there was a way to get comparable results using chemicals that are easier to obtain and dispose of.
Through some experimentation he found that sodium bisulfate (NaHSO4), which is a sodium salt of sulfuric acid, can easily be used in its place with great results. The chemical is typically advertised in hardware and pool stores as “Aqua Chem”, and can be had at a very reasonable price. When paired with the proper DC current along with a cathode, the sodium bisulfate easily anodizes an aluminum workpiece and renders it ready for coloring with RIT, readily available cloth dye.
We were impressed with the results, and when looking at [Ken’s] test pieces, it seems that the metal dyed with sodium bisulfate has a more uniform, less streaky coloring to it. It’s also worth mentioning that [Ken] has found it is fairly easy to etch the aluminum before anodizing using a solution of sodium hydroxide, which is great for individuals who prefer a more matte finish.
If this is something that interests you, be sure to swing by his site. He has a posted nice video overview of the process that may be of some help.