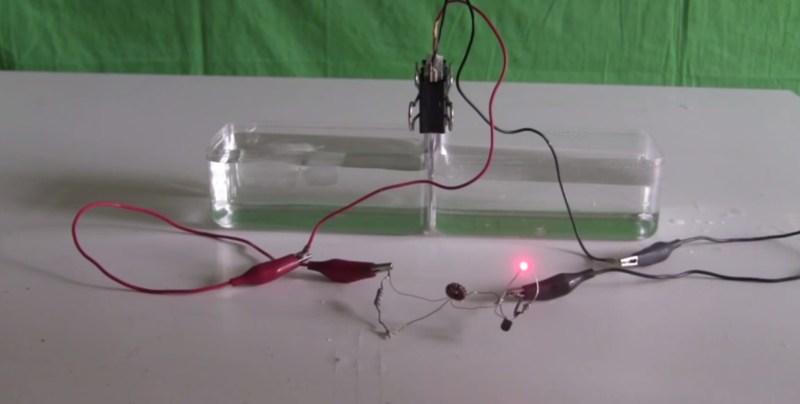
[Steven] manages to power an LED for 15 minutes using hot and cold water as a battery. He does this using the thermoelectric effect also known as the Seebeck effect, Peltier effect or Thomson effect. This isn’t particularly new; in fact there are commercial products that you can use to charge a cell phone using a small campfire or internal burner that works on the same principle.
What is interesting about [Steven’s] device is that he uses a salvaged Peltier device not meant for generating electricity, coupled with a home built joule thief circuit. In the video he describes how the joule thief functions and powers the LED using the small voltage generated by the Peltier device. The energy for the thermoelectric effect is conducted from a hot water bath through aluminum plates, through the positive and negative sides of the Peltier device, through more aluminum plates and finally into a cold water bath. As the heat energy transfers through the Peltier device a small electric current is generated and flows in two small wires coming out the side of the device. The energy generated by the Peltier device is stored in the joule thief and periodically dumped at a voltage high enough to forward bias the LED “on” for a brief moment. Technically the LED is flashing but at a frequency too high for our eyes to see. As the hot water bath cools, the LED goes from very bright, to dim, to off in about 15 minutes.
Not a very practical power supply but still quite the parlor trick. He wraps up the tutorial specifying that a TEG thermoelectric generator would be a much better choice for generating power and can handle much higher temperatures. You can watch the video after the break.















Stack three or four of them and use Arctic Silver between them.
Or you could use a homemade Zinc Air battery and power that led way beyond 15 min…
Is he trying to win a contest? I don’t think he is, so in that case this is still pretty cool and isn’t less interesting because there are better batteries. That’s not the point of the article.
Now compare this energy converter with a stirling motor attached with a magnet and induction coils.
As a chemist i admire the chemistry behind materials with high seebeck coefficients. It’s an interesting field, but in my opinion they will NEVER be used as an efficient energy conversion tool besides in Satellites and deep space probes.
Have you ever read about thermoelectric energy harvesters in car exhausts? The claimed power output at full throttle(!) is 700 Watts at best. A better use for hot exhaust gases is steam generation and gas turbines. BMW made such a “turbosteamer” a few years ago and claimed peak powers of 10 kilowatts!
Yup, there is a lot of power to harvest from exhausts. A 40% efficient car engine with 200 hp (149kW) will have 300 hp (223kW) energy wasted as heat. Large power plants could recover 60% of that heat, but in a small car I think 10kW is quite good.
http://www.autoweek.com/apps/pbcs.dll/article?AID=/20060227/FREE/302270007/1023/THISWEEKSISSUE
The recoverablility depends on the temperature differential. Large powerplants obtain high recovery efficiencies because the exhaust from the primary turbine is still very hot. They’re built to cascade like that on purpose, because any one stage cannot recover all the possible energy.
The exhaust from a car engine is not very hot. The average combustion temperature is something around 800 C and exhaust temperatures somewhere around 200 C under steady operation. The maximum efficiency obtainable from these is around 70% and 35% respectively, but since the Carnot engine cannot actually be built in reality, you’re limited somewhat to things like the otto cycle or brayton cycle etc.
So, the combined cycle powerplant is built with a gas turbine at the front, that combusts at nearly the melting point of its blades and extracts about 35% of the energy, and the exhaust gasses are as hot as what goes on inside a car engine, which then go on to turn multi-stage steam turbines that extract another 35% and pass lukewarm water out the back.
Part of the problem is that you want to keep the exhaust hot until you get to the catalytic converter. I always wondered just how much energy you could recover from a car engine if practicality was not an issue. Maybe use thermocouples between the coolant and the radiator, Thermocouples between the oil and the cooler, a turbine in the exhaust and maybe even pezio electric crystals on the motor mounts in in the exhaust. Totally impractical mind you.
So insulate the exhaust pipe from the engine to the cat, then recover the heat after. The cat contributes additional heat anyway.
Instead of a Joule thief I’d suggest to use this gem: BQ25504
It has temporary storage in a capacitor and overflow is sent to a battery or SuperCap. It can then access that as backup power source. The cold-start is 0.33V and can go as low as 80mV. It’s a QFN but worth some attention for this kind of projects.
Heh, might give me an excuse to dig out my collection of circa 1978 PNP germanium power transistors.
I have a box of them here, still in original packaging but no interest on *bay or locally.
Its also worth noting that with PE junctions the tricky part is keeping the hot side below the melting point of the solder or you will generate an expensive paperweight.
This often happens with refrigerator-scavenged panels because they are optimised for cooling and have a habit of melting down if the hot side goes much over 80C.
#include “ifoundthisouttheexpen$iveway.h”
The low melting point of solder is why I liked using the almost boiling hot water to provide heat for the hot side. The water’s maximum temperature is just under 100C/212F which is at the bottom of the maximum safe temperature range for the Peltier module. It provides an easy self-limiting temperature at coincidentally the right temperature. I didn’t have to worry about damaging the module, while getting close to maximum performance.
Could you, in theory, create some sort of thermal pump that boils water on a wood stove and recirculates the water by passing the steam through a loop that is cooled by a fan that also cools the peltier at the same go?
That’d be a pretty cool camping light.
like this , http://www.survivalschool.com/products/power_generation/Liberty_Generator_for_Coleman.jpg
So how high of a differential do you need?
I don’t know. I was thinking afterward that I should have put some thermometers in the containers. Silly me.
I was wondering if a black aluminum plate exposed to the sun as the hot side and an insulated metal pole going into the ground as the cool side would work. I do live in south florida so heat is not an issue but cold can be hard to get.
I think a sheet of flat black painted metal would get plenty hot enough, given how a dark colored car in your hot Florida sun can get too hot to touch. Also, a certain depth underground stays the same temperature all year round. Here in the Ottawa, Canada area around 5 feet down it’s always around 55F. That information is online somewhere.
I also found this out the expensive way., but there’s a workaround. Was able to solder the leads back onto the junction using some woods metal. Made up about an ounce of woods metal from some bismuth fishing sinkers, lead fishing sinkers, and tin ‘lead free’ solder, and poured it into some plastic tubing. Strip off the tube when the metal cools and you have woods metal wire solder. Melts at about 220 degrees F.
“use this gem: BQ25504”
I was going to suggest the same thing, I have a half dozen YX805’s which are similar.
It only requires a single inductor..
“germanium power transistors”
very leaky and the leakage changes drasticly with temp.
MOSFETS (with proper gate drive) work much better.
The joule thief chips mentioned have all that built in.