While researching copper plating graphite for a project, [Dave] stumbled upon a blog post illustrating a brilliant approach to metal plating 3D printed parts.
Our pioneers in this new technique are [Aaron], who runs a jewelry business, and [Bryan], a professor of Digital Media. By mixing graphite powder into an acetone solution, it is possible to make a kind of graphite paint that sticks extremely well to ABS plastic.
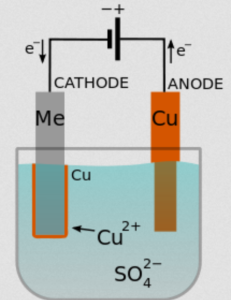
Using the graphite painted part as the cathode, and a chunk of copper as the anode, it becomes possible to electroplate the part with a variety of electro-forming solutions. In the first test (seen above), [Bryan] uses a Midas Bright Electro-forming Copper Solution (copper sulfate solution).
The electroplating process is the same as any other metal plating hack we’ve covered, but if you’re a bit rusty on your chemistry knowledge, we’ve thrown in a little easy-to-read diagram to refresh your memory.
As the current flows through the solution, dissolved metal cations form a coating on the cathode, creating a coherent plating effect — this process is called electrodeposition.
The results are quite impressive — we can see how [Aaron] makes some pretty cool jewelry combining 3D printing with this technique!
[Bryan Cera] is a Wisconsin maker who teaches digital media at the Cardinal Stritch University. His studio work focuses on the intersection of art, engineering and design.
[Thanks Dave!]
















Maybe there is some way to leverage this to print circuit traces???
They use conductive inks now.
I guess you would have to connect all the traces somehow in order for it to work?
Raise the area you don’t want copper on. After plating you can sand down all the raised areas leaving the copper ‘valleys’ intact.
Intriguing! But you won’t be able to use graphite as your base. I would let go of the copper trace easily.
Why not mix powdered copper with acetone and skip the graphite step?
They actually just make a paint that is copper powder. It is just a bit pricy for me, so I wanted to create a different solution. It isn’t the best but it works. I was told that it is possible to leech copper from copper sulfate. That is my next step to figure out a better paint.
Hi Aaron,
last time, after etching PCBs with H2O2+HCl (muratic acid) I precipitated Cu by throwing aluminium scrap into the diluted solution. this caused small copper (Cu) crystals to grow.
Possibly a good way
a) to get rid of the environmental toxic copper solution and
b) to obtain copper powder “for free”
Regards,
Jérôme
Because you would probably end up with a high resistance traces due to the copper particle size. Besides it still wouldn’t stick to a PCB as Acetone does bugger all to the epoxy that the PCB is made out of.
Maybe you could print the traces in thick layers and then place the board facedown in a very shallow bath of the solution, rubber-stamp style. I’m not sure if you’d have problems with the solution wicking around features though. Sounds like trouble.
There are other intriguing applications of this process though – for instance, applying the same electroplating procedure over the conductive inks currently used, in order to grow robust copper tracks over the fragile and high-resistance ink. Sounds like an area which needs some research.
Maybe selectively energizing the tracks would be a good way to do it? I wonder if you could accomplish variable deposition thicknesses to get high-current vs small-signal traces?
Perhaps print the part. Mask off the areas that are not to be traces. Then plate the rest.
Or better yet (but trickier and longer)
Print the part. Plate the whole part. Print over the traces. Remove the excess plating. Reprint over the edges to seal in the traces.
I’m thinking perhaps a Christmas tree. Drill holes and “plug in” some flashing LEDs and maybe use some of that conducive trace glue instead of failing to solder. Or perhaps print pegs or insert pins that can be plated over and connected wire wrap style.
I’m getting tired of hearing ‘conductive ink’, shouldn’t it be called ‘conductive filament’ or ‘solder’ if that’s what it is? (it certainly looks like it)
Um … conductive ink is not solder, rather a dispersion of silver or copper particles, meaning you can make working PCBs on an inkjet printer. See:
Park, B. K., Kim, D., Jeong, S., Moon, J., & Kim, J. S. (2007). Direct writing of copper conductive patterns by ink-jet printing. Thin Solid Films, 515(19), 7706-7711.
OK, so how do you solder your parts to that when the plastic under it melts?
The better solution is to use the standard photo etch process on standard copper clad fiberboard.
that might be the better solution for PCB right now, but not for making shiny coppery 3D prints.
The deposition should only occur on conductive surfaces, so nothing that is not coated in graphite should be coated. What if, instead of painting the graphite on by hand, a graphite-containing filament was used along with a dualstruder? Although the graphite filament is advertised as being conductive enough for circuitry, it doesn’t conduct very well at all, especially compared to copper.
The problem I see is that the copper deposition might not form a crisp line between conductive and non-conductive parts. This might put a limit on how small the traces can be. The traces might instead be limited by the smallest diameter nozzle that can extrude graphite filament, which is notoriously finicky and prone to clogging.
Or maybe you can electroplate THEN etch (then print over that). Not as straightforward, of course.
Copper sulfate is used as a fertilizer available at Tractor Supply Store. 15 lbs 90 bux,ouch. Used to be able to purchase in smaller quantities for sewage tank treatment to kill roots. I looked this past weekend but could only find products that boasted being CuSO4 free at loews and Home depot.
Was going to print in conductive ABS and then plate with copper. Experiment with creating resistor/capacitor all in one. Has any one used conductive abs and electroplating to create electrical components?
Should also be possible to plate from copper dissolved in Muriatic acid (HCl) available at hardware stores for cleaning concrete.
Not sure where to put this…
http://techref.massmind.org/techref/pcb/etch/CuCl2.htm discusses cupric chloride, which I believe generates solid Cu2 in e.g. an air bubble bath. The Cu2 freed in this way might be electroplatable. Any chemist around?
Copper sulfate isn’t a fertilizer, but a poison. It is used as a herbicide, fungicide and pesticide.
It is both a fertilizer and X-cides. Seemed contradictory to me as well, but its function is application dependent. You can also use it to grow pretty crystals for science fairs. It used to come in chemistry sets 30 years ago. Point is you can purchase it for about half the price from TSC than from a chemical supply house. The assay indicates 99% pure.
Whether it functions as a fertilizer or biocide is dose-dependent. It’s present in many fertilizer blends, in very small quantities. Original Miracle-Gro is one that’s bit heavy on the CuSO4, giving it the characteristic blue-green color.
Dose is the key hullo Paracelsus, copper sulphate (in Western Australia) is used in feed for milking cattle to increase production as far as I know to shift gut bacteria equilibrium and potentially to stimulate enzymes for milk production in the mammaries too. I’ve been researching copper post grad part of Food Science) regarding effects as long term nootropic in conjunction with several other adidtions, our ancestors were ingesting approx 300x (!) what we get now on average daily basis and many without ill effects, its a more powerful antioxidant than Vitamin C but can be exploited by cancers, humans have great difficulty metabolising iron if our intake is deficient (as suggested 79% of US population are below RDI) as the pathway is through copper based enzymes. This affects ability to handle complex thoughts Eg allowing a brain receptor (NMDA) to die from deficiency…
Look for ‘Bluestone’ in the fertilizer section. It’s used to spray tomato plants
“if you’re a bit rusty on your chemistry knowledge” – sure am, I can’t remember any element “Me” on the periodic tables I learnt.
Me is the Chemical Symbol for the element Métallon on the new Periodic Table, just another one of those Executive Orders nobody knew about except for the commander-in-chief..
http://ic.pics.livejournal.com/hackrabbit/108910/26539/26539_original.png
Here’s a refresher on the updated version of the table
Well I am going blind to, still don’t see it!
It’s not difficult, you just have to Look Around You,
I cracked up so hard at that..
I had to LOL at “Manganesium” xD
Isn’t this originally from the BBC series “Look Around You”?
!
!!
This exact process can be used to create Plated through holes on home-brew PCB boards. The trick is that you have to drill and plate the holes before you etch the traces. This means you have to apply the resist after you drill and plate the board because you need the holes to all be connected during plating.
H3 Labs has been investigating using a traditional tin oxide base layer process for plating plastic, but we’ll definitely try this… it’s much simpler: no ball mill required, no vacuum chamber needed for depositing the initial layer.
Would Bryan and Aaron be able to share more information on the graphite paint?
It is just acetone and powered graphite. I don’t have an precise measurements, they are not really needed. You just need enough acetone to create a gooey solution.
I would start with the graphite and add acetone until it is all ‘wet’. Then you may add more acetone if it’s too clumpy and hard to apply.
A lot of plastics are soluble in acetone (obviously this is one of them) so it’s the actual plastic that becomes the glue as it starts to dissolve. It then captures the graphite as the acetone evaporates and the plastic hardens again.
If you know the weight per ml of graphite, the surface area of the print and the thickness of the graphite coating you want then you can accurately calculate how much graphite to use (plus some wastage). The amount of acetone is less critical as it evaporates anyway.
Our Cosmichrome product is use by rapid prototyping shops to metalize rapid prototype parts. Although we also manufacture electroforming chemicals Cosmichrome is much easier to do. Cosmichrome is applied like paint in a paint booth. General Motors uses Cosmichrome in their design shop for all their prototypes. No vacuum chamber is required. Just a paint booth.
GM even coated an entire Camaro with it a couple years ago. The substrate really doesn’t matter. Wood. Metal. Plastic. Foam. Cosmichrome won’t affect the tolerances of your parts like electroforming. http://goldtouchinc.com/cosmichrome-spray-chrome/
There are wacky ways used for electroplating plastic (i.e. nonconductive) parts that don’t use graphite paint. Graphite paint is usually the first thing people think of, but there are many other ways of making the surface conductive. A popular technique is to deposit a layer of electroless copper on the surface first, which doesn’t require an electrochemical cell. As far as I remember, this involves something like dipping the entire part in a palladium chloride solution first, then into a copper salt solution. A displacement reaction occurs which leaves a very thin layer of copper behind. Thereafter, it’s back to normal electroplating.
Google “electroless plating” and there’s plenty comes up.
I’ve seen parts printed by Objet’s machines which were subsequently chrome plated and polished – friggin’ amazing.
The idea has been around for over a century and has been used to produce the statues in quiet a few famous monuments. There are some very informative texts on the various methods and chemistry to be found on archive.org
vinegar and a copper scrub bud makes a great -FREE- copper plating solution.
I saw an instructible using peroxide and vinegar but you would not believe how hard it was to find cheap copper wire scrubbers. I could only find cleaning boy or something scrubbers online and i am doubtful over the copper used in some of the online stuff. I think it can have a weird coating. Otherwise I had to get some from expensive, squeezer homewares shops for like 50 bucks for 2 bucks worth of extruded copper. Also old transformer wire minus the enamel and an old mac laptop ps had a big copper shield inside.
Check your local dollar stores, most have copper scrubbers very cheap.
Depends on where you live. Around here, the 100% copper “Chore boys” are either not carried, or hidden from view. They are alternatively used as a filter of sorts in meth pipes.
Forget the scrubbers just add metallic copper from any handy source best in my experience is stranded wire (high surface area to react) but i’ve made acetate plating baths from pipe, 14guage romex, even pennies (more trouble than it is worth and they have to be all copper which new ones arent).
A friend of mine runs a business selling an easy to use water soluble paint designed for electroforming/electrodepositing on non-conductive materials. I have used it and it works extremely well without needing any solvents at all. http://www.safer-solutions.com/safer-solutions.com/Safer_Solutions.html Lots of really great info based on real world experience there as well.
Guys. On market now is electrical conductive ABS filament. So you could avoid graphite step in here
I found out about this filament about a month after I started working on this. I plan on buying a spool soon to play with!
This type of electroplating (more or less) has been done for quite some time in industry. Here is a general “how-to” guide that talks about the basics.
http://www.azom.com/article.aspx?ArticleID=525
How about doing the opposite and growing an object inside a negative mold?
just a few hundred million layers you mean?
One can always grow a thin layer and fill the object with plaster or resin/wax for rigidity.
Point being that the dimensions become more controllable because you’re growing the object from the outside in, rather than adding material on top.
I like what Dax is thinking. Negative mold means that you aren’t trapping a layer of graphite paint just beneath the plating.
You don’t need any paint at all if your plastic is electrically conductive, like, if you mix in enough carbon to it.
Nice – but what’s the big deal? It’s a classic electroplating/electroforming process. We are using the “hot bath” varitey for 100+ years in our modelmaking/prototyping dept. and use the “cold bath” variety for 10+ years now since STL parts are temperature sensitive…
My sister in law makes jewellery and has used this process many times. My brother has plated a few prints for me a while ago, and resukts were great! I used some copper conductive paint that you can get from electroplating companies. Results are good, however making sure your print is a nice one first will leave much better results.
Should add that the graphite should be ground to a fine powder, as it looks like the blobbyness comes from the lumps… But this is a good option.
This could make some bitchin tabletop miniatures, figurines, and the like. It’d be neat to electroplate, say, just the armor, or the sword, rather than having to use metallic paint, or the black magic that is non-metal metallics (ie painting the reflections the hard way).
if you print conductive (PLA oe ABS) filament, you should be able to have a felt ring around the extruder, lightly touching the extruded material. Soak the felt ring in electroplating solution, and run a current from the ring to the nozzle. A thin copper deposit will form on the print, much reducing the resistance of the the material a lot.
Reblogged this on sansthelight and commented:
I have been slowly working on a 3d printed chess set. I probably won’t try this with that set, but it is nice to know the option is there.
Find here Electroplating Chemicals manufacturers, suppliers & exporters in Surat, Gujarat. Get contact details & address of companies manufacturing and supplying Electroplating Chemicals in Surat, Gujarat.