The tech is nothing new, but did you know you can make your own graphene using your DVD burner? No seriously — all you need is a light-scribe compatible DVD burner and some graphite oxide.
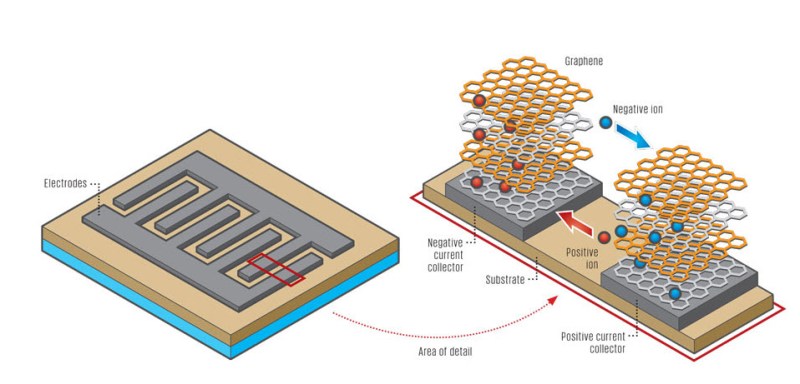
It’s pretty simple. By placing a thin film on top of a DVD (or any plastic CD shaped disc), and coating it with graphite oxide, you can literally print patterns of graphene using the laser in your DVD burner. By making the shapes shown above, you can introduce an electrolyte and turn the whole thing into a supercapacitor. Albeit, a tiny super capacitor. But — you can print hundreds of them on a DVD in less than an hour.
We’ve covered this before a few times now, but that doesn’t make it any less interesting. We’re still waiting for someone (one of you guys!) to do a project that actually makes use of graphene! Hurry up!
[Thanks for the tip John!]















Planning on it…
What exactly does ‘tiny super capacitor’ means? It is definitely not tiny in the means of physical size, so it must be tiny in capacity, although then why is it called a super capacitor in the first place? Please someone explain.
It’s a silly technique, only really unique because no seperator is needed. There could be a few niche uses for laser annealed and reduced graphene oxide but most often it is reduced in bulk for larger applications.
Exotic expensive electrolytes are required for higher voltage capacitance that directly effects energy density.
Quaternary ammonium salts and deep eutectic solvents are whats needed for a good electrolyte. You can make first gen stuff with aluminum chloride and urea but it is really only a novelty.
The best ones to date are asymmetric, anyway and built in a roll or plates using a commercial separator. I am still playing with a MNO2 GO(graphene oxide) lithium hydroxide electrolyte super capacitor, but it is definitely not as easy as these publications make it out to be.
If you want to make some this is your best bet for ease of creation.
http://www.researchgate.net/publication/222874161_A_novel_hybrid_manganese_dioxideactivated_carbon_supercapacitor_using_lithium_hydroxide_electrolyte
correction thats MnO2 one too many capitals there
Absurd!
[youtube http://www.youtube.com/watch?v=WAOxY_nHdew&w=420&h=315%5D
This /\ coming from someone blogging this dribble http://www.cyclopsjack.com/2015/09/ahmed-mohamed-and-his-ominous-clock.html
It’s ok, I understand it went about 200 meters over your head.
Drivel, not dribble.
http://www.nytimes.com/2008/09/19/opinion/19iht-eddas.1.16308269.html
Yes, total dribble…drivel (language such a burden to actually understand). The solid state transistor changed the world and nobody saw it coming. If there had been message boards back in the 60s, they would have looked much like this one nothing to see here move along…. A bunch of Luddites pooh-poohing away. I lay one finger on my right nostril and snort a booger in your general direction! I suspect one day you might look back on what you wrote today in genuine humiliation. You might wonder how you could miss the single biggest discovery in the history of forever. But I doubt any of you are introspective enough to know you shouldn’t pass gas at church.
Any guess as to the energy density of this technique? I’m thinking for powering something stationary where the energy storage can be huge and heavy as long as it is cheap.
Not nearly as good as batteries, and the super-capacitors generally have leakage current as well as a high ESR(equivalent series resistance) with cheap electrolyte/seperators. The problem really is aqueous electrolyte, every time you charge its going to off gas hydrogen and oxygen. They are really only goo for high power density applications over high energy density applications.
Here is a best case scenario graph. If you have everything right you can expect to be on the top of the chart of the given category.
http://www.seas.ucla.edu/~pilon/images/supercapacitors/Power%20vs%20Energy.png
You probably want to buy some lead acid deep cycle batteries, they will cost far less than the materials and time it will take to assemble a super capacitor, If you treat them right they will last a very long time. If what you really want to do is build something, build a battery. Click on my name, I am almost done with an open source one, I am waiting for a few more materials currently.
Super capacitor is a reference to it’s electrical behavior, not it’s capacity. https://en.wikipedia.org/wiki/Supercapacitor
Yes, we kinda do know http://hackaday.com/2012/12/21/making-graphene-with-a-dvd-burner/
didnt read the whole post, so you know this was covered – now Im confused, is it like a reminder post ?
Yes.
Slightly different technique. You had to burn two platters, use an electrolyte gel, and a semi-permeable membrane between the two graphene sheets.
This new technique burns both poles of the capacitor on one DVD platter. They use an etching technique to make a gap between two zones. You then slather it with an electrolyte gel, and seal it. What this does is save you money by not buying the expensive 3M semipermeable membrane material.
I like it, and I am happy for this post. This technique makes these devices much more accessible to home tinkerers.
And this, which is older almost identical to the current one: http://hackaday.com/2012/03/20/print-your-own-supercaps/ :P
*older and almost, sorry, missed a word there.
I have this perpetual motion machine design and I wonder if I could leverage this technology to zero-point the climate denialists… or something
If it is 3D printed from recycled materials… Then, yes.
Just make sure it doesn’t look like a bomb.
Sounds like a good Kickstarter. Where do I throw my money?
I have actually been working on a modified version of the heptane-water interface method of making graphene for the past year or so, more so stocking up my ‘secret lab’ with chems and equipment. No, it is not for the home tinkerer as it required purchasing an ultrasonic homogenizer.. the alternative would be the soap+blender method for a simple at-home low-cost just-fooling-around sort of guy. Indeed you would still need to work out an electrolyte as well, preferbly gel/solid now-a-days…
Lightscribe is still around? I had no idea.
I used hummer method to prepare graphene oxide, initially with a brown color and poured it in to a closed flask and leave the solution for some time. The solution settled down, water comes up, but the color of solution turned black. Please guide me why is this happening?
What should i do know?