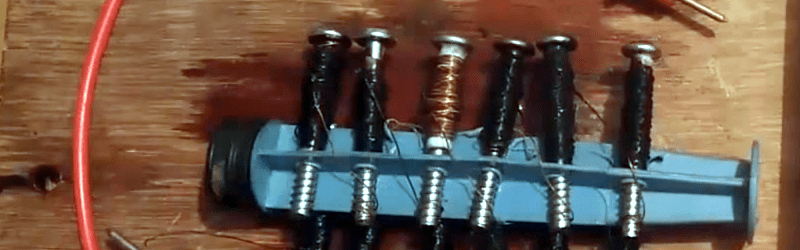
Volta’s pile — the first battery — was little more than silver and zinc discs separated by paper soaked in salt water. A classic classroom experiment is to build a pile from copper pennies, tin foil, and vinegar or lemon juice. [Omars2] has a different take on this old experiment. He creates a 9V battery using some zinc screws, copper wire, and salt water. There’s a video of the battery, below.
A syringe piston serves as a substrate for the cells, and each cell is just a screw with paper wrapped around it and then 35 turns of copper wire on top of that. The battery is soaked in salt water, although we suspect vinegar or lemon juice would work even better. Heating the electrolyte is also a good idea.
The theory is pretty simple: The salt dissolves into positively charged sodium ions and negatively charged chlorine atoms to form the electrolyte. The cathode loses electrons into the solution, leaving it positive. The other electrode — the anode — collects electrons, so it has a net negative charge. The difference between the charge on the two electrodes creates a potential difference. When you close the circuit, electrons flow from the anode back to the cathode, causing an electrical current.
If you’d rather make the penny version of this, it’s been easier in the US since 1982 when pennies became copper-covered zinc. Grinding the copper off one side of each penny allows them to act as both electrodes. Old pennies don’t have a zinc core, so to use those you’d need another metal like tin foil.
If you want something more impressive looking, try a cola can battery. If these aren’t exciting enough for you, consider a nuclear battery.















c/dissolves/dissociates/
Isn’t it s/EXPRESSION/REPLACE/ ?
Does it work on vi? Which is by far the best editor in the world. That’s a fact, not an opinion. Very objective. Proven, by the way.
Where was I? Ah, yes: Isn’t it s/ instead of c/?
‘vi’ can be heavily customized, so I guess one can customize ‘vi’ to substitute text using ‘c’ instead of ‘s’, but why talking about ‘vi’, isn’t it ‘s/regexp/replacement/flags’ a ‘sed’ command?
“Rechargable” zinc/copper battery?
Wait for the next nova explosion and collect new zinc and copper. Tada: Recharged.
Even oil is renewable if you can wait for long enough.
EUR cent coins are copper clad iron. They should also work.
grinding the copper off of one side allows them to act as both electrodes pre-shorted to each other.
The outer layer is copper, the inner layer is zinc, and they are separated by a creamy nougat center.
The two sides just must not be part of the same cell, i.e. not touch the same volume of electrolyte. So you have to grind over the edge and at best seal the rim with some nonconductive stuff (glue, silicone)
That video’s terrible. It’s got horrible music, no commentary or explanation. He claims the battery is “rechargable”, I’d love to know how. And then underneath there’s a link to his free-energy device. Yup the over-unity type. There’s some waffle about magnetism in there.
The guy’s an idiot. You must be able to find some schoolkids somewhere who can make a battery without being convinced they’ve bent the laws of physics.
Sad. Yet another blogger acting like a God and spreading faulty lessons:
“Hi!
In this instructable, you will learn how to make a powerful 9V rechargeable battery from iron nails and copper wire.The battery is rechargeable like any other normal battery and is really simple to make.
For complete understanding of the project you must watch the video.”
First, WTF?! Such a start raises a few red flags. But OK, whatever.
Then, a 9V (8.47V measured in the video) out of 12 Fe-Cu elements?
Blogger says Fe-Cu (0.8V/cell), Hackaday says Zn-Cu (1.1V/cell), but voltmeter says neither, most probably Mn-Cu judging by the 1.3V measured on one of the cells. The rest of the cells voltages are all over the place, between 0.3V and 1.2V. At a closer look, the blogger literally burned the cells with fire. That might be an explanation. Another two cells are completely short-circuited, they have 0V. Still, it was good enough to be further touted on Hackaday.
Yes, it was a nice try, but don’t act like a teacher. I understand that failures are good for learning, but only when we realize we have failed. Otherwise we will become delusional.
TL;DR no state of the art here, the art museum must be somewhere else.
https://www.youtube.com/watch?v=gur4RFOH2x4
:o)
Fake. No way those batteries power that motor at that speed. Also there is a cut in the video at the point where the motor is connected.
Oh Hackaday, you silly sausages. This is all a load of woo :D