Of all known metals, mercury is probably one of the most famous, if only for its lustrous, liquid form at room temperature. Over the centuries, it has been commonly used in a wide variety of applications, including industrial chemical processes, in cosmetics, for telescope mirrors, thermometers, fluorescent lamps, dental fillings, bearings, batteries, switches and most recently in atomic clocks.
Though hardly free from the controversy often surrounding a toxic heavy metal, it’s hard to argue the myriad ways in which mercury has played a positive role in humanity’s technological progress and scientific discoveries. This article will focus both on its historical, current, and possible future uses, as well as the darker side of this fascinating metal.
Shiny and Useful
Mercury has been highly prized for its use in art and decorations. It’s historically known to have been used in mercury fountains — exactly as it sounds, these were artistic fountains using mercury rather than water — with the most recent example being Alexander Calder’s 1937 Mercury Fountain. Yet for thousands of years, from the Mayans (Temple of the Feathered Serpent in Teotihuacan) to the Egyptians and the Chinese (Emperor’s Qin tomb), mercury was held in high esteem, with many considering it to hold special properties in addition to its remarkable physical properties.
Unfortunately, this led to it being used in medicine, especially in China and Tibet, where it was thought to prolong life, heal injuries and generally improve one’s health. It is rumored that a mixture of mercury and crushed jade given as an immortality mixture was what killed Emperor Qin. Alchemists considered mercury to be a Prima Materia (First Matter) from which other metals are derived.

Clearly more practical was the discovery around 500 BC of amalgams (from medieval Latin amalgama, “alloy of mercury”), the mixing of mercury with other metals which led to its use for dental fillings in China before 1000 AD and in Europe around 1528 AD. Much like the Chinese amalgams back then, dental amalgams today consist of mercury and a metal alloy of silver, tin, and copper.
While polymer resins are being used more commonly instead of amalgam in dentistry, amalgam remains superior in terms of longevity and durability, except for situations where the restored area would be directly visible (polymer resins being white), or the hole in the tooth is fairly small. Here polymer resins are the preferred material.
Despite the scares about mercury poisoning from the elemental mercury in amalgam dental fillings, studies have shown that the amounts of mercury released is low enough that it should pose no health risks. Regardless, dental offices in the EU are required to treat amalgam waste as hazardous waste. US dental offices are facing similar measures, but flushing the amalgam waste down the drain is still common practice.
The Distinction Between Useful and Hazardous
Even outside of dental amalgam, mercury manages to provoke fierce debates about its uses and perceived dangers. One of these involves the many organic compounds that contain mercury, the so-called organomercury compounds. This group includes methylmercury (commonly found in fish like tuna and salmon), ethylmercury , dimethylmercury, diethylmercury, and merbromin.
Commonly used as a preservative agent due to its antiseptic and antifungal properties, thiomersal is regularly used in everything from vaccines to ophthalmic (e.g. eyedrops) and nasal products as well as things like tattoo inks and mascara, where long-time sterility is essential. In the body, thiomersal is broken down into ethylmercury, which is significantly less dangerous to the body than methylmercury. While refrigeration is an alternative to thiomersal, it requires an uninterrupted cooling chain, which can be problematic in some areas, leading to the use of contaminated vaccines.
In the US, fears about the ‘mercury’ in vaccines (related to conspiracy theories involving autism caused by vaccines) led to thiomersal being removed from most vaccines despite a lack of scientific evidence for doing so. Due to a lack of data on ethylmercury’s effect on the body in the 1990s, the data for methylmercury was used instead. Later research showed this to be a wrong equivalence, instead showing just how much more harmful methylmercury is.
Incidentally, the same conspiracy theories that led to the removal of thiomersal from most vaccines is linked into a more grand conspiracy theory about autism being caused by environmental toxins, including lead, mercury and other heavy metals. Chelation therapy is supposed to remove these toxins. This is however strongly recommended against as it is not an effective treatment and can lead to kidney and other potentially fatal damage.
Don’t Eat That Fish
Methylmercury is the most common form of organomercury, as it’s formed from inorganic mercury by microbes that live in aquatic systems. The resulting methylmercury is readily consumed by algae, which in turn are consumed by ever larger fish and other aquatic organisms in a process called biomagnification. As a result, the consumption of fish is the largest source of methylmercury and mercury in general for the population.

Mercury poisoning became well known due to the sudden outbreak of the then new Minamata disease in Japan, which turned out to be caused by the release of methylmercury into the environment from chemical factories, ending up in aquatic organisms that the local population would then catch and consume. In Japan this disease would cost 1,784 lives of 2,265 officially identified victims. Other nations experienced their own outbreaks of this disease.
Even without deliberate spills of methylmercury or its precursors, the amount of mercury in the environment is such that for fish species like swordfish, tuna, cod and pike one should not eat more than 170 grams of it per week, to avoid an unhealthy bioaccumulation of mercury in one’s body. Some places like Florida’s Everglades end up acting like scrubbers for mercury that is released in the air, severely raising local mercury levels there, with the advice being to never eat fish caught in those areas.
Mercury Today and Tomorrow
It is hard to think of a world without mercury. Whether it’s in dentistry, industry, laboratories or in astronomy, an essential role is played by mercury in some fashion. In astronomy especially, mercury essentially enables liquid mirror telescopes, which provide a highly effective and low-cost alternative to expensive and fragile glass mirrors. Mercury sees common use as an electrode in chemistry and in X-ray crystallography studies of proteins in structural biology with the multiple isomorphous replacement (MIR) approach, even as its use in more mundane tasks such as diffusion vacuum pumps has diminished over time, it still remains relevant there, as recently covered on Hackaday.
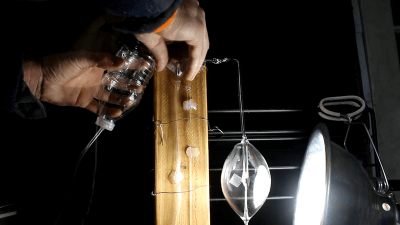
One of the most exciting new applications for mercury in the near future is that Jet Propulsion Lab’s Deep Space Atomic Clock (DSAC). The exciting thing about the DSAC is that it essentially takes the accuracy of a rubidium-based atomic clock (AC) and stuffs it into a package many times smaller. This is all courtesy of the properties of mercury ions which allowed for such a level of miniaturization, allowing it to be used in weight-sensitive applications, such as space probes and satellites.
Mercury in Space
The obvious advantage of the DSAC project is that the high clock stability improves the on-board time-tracking and thus navigation and communication abilities, which would be ideal for deep space missions. This is further detailed in a 2012 JPL paper on the project. It describes the crucial role the onboard timing source has on deep space navigation when it comes to forming multi-way coherent Doppler and range measurements. The essential benefit is that a spacecraft can do more by itself, with higher accuracy and higher useful data rates across the network.
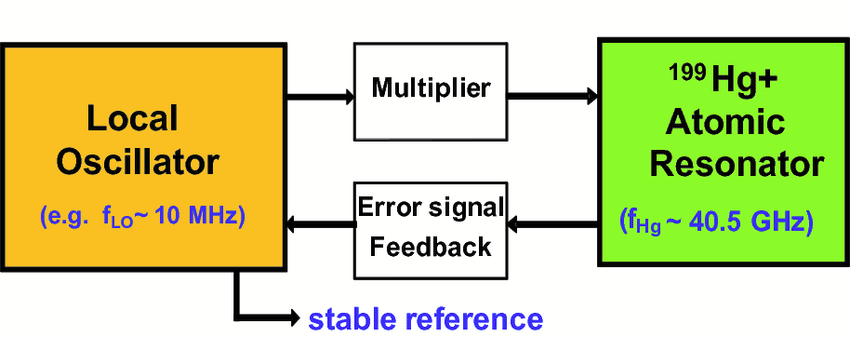
Mercury is similar to rubidium in that it has a hyperfine transition that emits a very precise electromagnetic signal. Much of the miniaturization is enabled by the fact that in a microwave-driven atomic clock (like in current rubidium ACs and the DSAC) the frequency that is required to drive the clock also determines the dimensions of the oscillator which drives the clock. Whereas a rubidium clock uses a paltry ~6.834 GHz, mercury-199 uses 40.5 GHz.
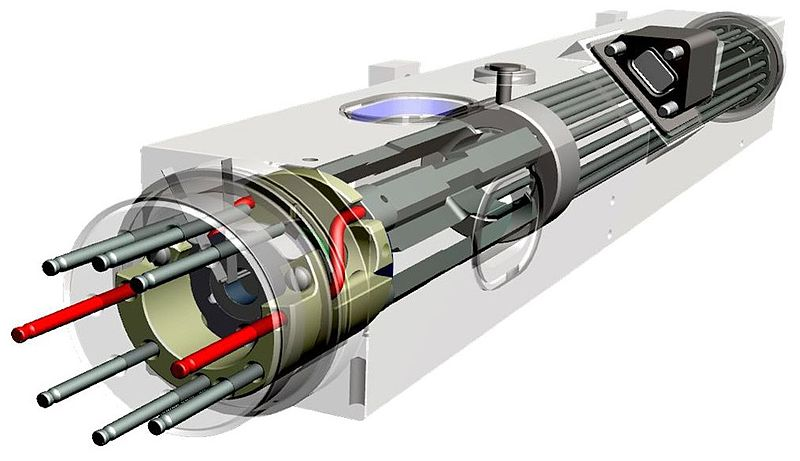
At those higher frequencies, the required circuitry and other components can be made much smaller, resulting in an atomic clock (current version) that’s a mere 29 by 26 by 23 cm at 17.5 kg, yet show no more drift than about 1 microsecond in 10 years of operation.
A DSAC prototype was launched on June 25th 2019 from Kennedy Space Center on a SpaceX Falcon Heavy rocket, as part of the Orbital Test Bed (OTB) satellite, which hosts four additional payloads in addition to the DSAC. NASA activated the DSAC prototype on August 23rd, with the entire mission expected to take about a year.
Being the Right Atom, in the Right Place
Throughout history, mercury has been a bit of a celebrity metal. In addition to its highly unusual liquid state at room temperature, it has enabled many areas of science to progress in ways that would have been difficult without mercury. Whether one looks at diffusion pumps and mercury thermometers, its myriad roles in chemistry and industry, the preservation of vaccines and similar substances, it’s hard to think of a material which has impacted human civilization more in ways that are subtle but ever-present.
Now it appears that mercury will be with us on our journey to the final frontier as well, keeping our space probes and possibly crewed space ships safe as they travel to Mars, Venus, and beyond. Here’s to a long, healthy relationship with a really special metal.














“it’s hard to think of a material which has impacted human civilization more in ways that are subtle but ever-present.”…
If you ignore carbon
That mercury atomic clock looks really interesting. I’:e not seen it before.
Mercury is also the critical component in a rare and dangerous technique in horology and conservation called fire gilding, which uses gold and flame to burn off mercury from an amalgam of the two, leaving a very fine layer of gold gilding on an object.
Fire gilding drove many gilders slowly insane from the mercury fumes, similar to haberdashery, which many know of, where mercury was used to mat down beaver fur for tophats.
Fire gilding in horology makes an appearance in a recent podcast series (https://stownpodcast.org/).
Yes- heard that when it came out. That was in my mind when commenting. Incredible story, that one.
The hatters used a mercury compound soluble in water (and fats).
The Interwebs repeat the usual and fasle-ish ideas about mercury everywhere I check. For example, Google quotes a wiki page “The origin of the phrase, it’s believed, is that hatters really did go mad. The chemicals used in hat-making included mercurous nitrate, used in curing felt. Prolonged exposure to the mercury vapors caused mercury poisoning.”
But mercury(II) nitrate is a salt that in water is an acid salt, and felt was priocessed in it by hand and machine without protection. Absorption through the skin and by mouth must have vastly exceeded any vapors. It seems everyone KNOWS mercury vapor is bad and then write as an authority with assumptions about real situations. Fascinating.
Maybe someone knows the vapor pressure in solution. Anyway, the ions don’t want to leave the solution and I bet the Hg+ that get kicked into the air are few and far between.
The interesting list would be “elements that have not affected my life”.
Unobtanium?
I wouldn’t really call carbon’s role subtle.
Hi
Is carbon a “material”? Which allotropes of carbon, like graphite, diamond or carbon nanotubes are you thinking of?
Ok:
the article refers to “organomercury compounds” so a fair comparison between the “wonderful” attributes of mercury and carbon should include “organo-carbon compounds” :-)
if discussing mercury alloys (amalgams) it is only fair to point out that carbon alloys such as steel and tungsten carbide are a bit more useful and have made a far greater impact on man. :-)
If we are talking about clocks what about “carbon dating”.
What about the use of carbon brushes as a way of supplying power to motors.
What about graphite used as a solid lubricant (see locks)
What about zinc / carbon batteries
What about graphite crucibles and moulds.
What about graphite gland packing (see plumbing)
What about pyrolytic carbon with its amazing heat conduction ability (see ice knife).
I’m no expert but I’m sure there must be many more things that I’m not even aware of :-)
> studies have shown that the amounts of mercury released is low enough that it should pose no health risks.
studies have also show the opposite.. depending on who finances the studies!
flour and mercury are poisonous, and yet somehow safe to put in your mouth?
Flour is very safe to put in your mouth, especially if you mix it with yeast, water and a pinch of salt.
Fluor is a highly corrosive gas that would be very unsafe to put in your mouth.
Fluoride on the other hand is quite safe to put in your mouth.
In the same vein, mercury is not the same as thiomersal.
AFAIK flour is used to bake bread and not considered dangerous, except that excessive consumption can lead to adipositas.
If you think of fluorine, yes that is toxic. Too much fluoride is also toxic, but a small amount is helpful. Not the only substance where a little is good and too much is bad. Even ingesting too much water is deadly – about 14 liters in a short time have already been fatal.
Also selenium: a small amount is necessary for your immune system to function properly, a little more is toxic. Fat soluble vitamins are another example.
“AFAIK flour is used to bake bread and not considered dangerous”
Flour finely dispersed in air can be very dangerous.
I think at some point mercury was used as a treatment for syphilis, wasn’t it? Not sure how that’s supposed to work. Was the mercury ingested? Injected? Applied topically?
Also, I think one of the current uses for mercury is in gold mining/panning where it’s used to boil off other material leaving only the gold. As another commenter has mentioned above this practice is very hazardous.
The mercury was injected into the urethra with a rather crude syringe.
It was also dispensed in pill form.
Dr. Ehrlich’s Magic Bullet involved arsenic.
It worked as an antibiotic – maybe – when used externally. You had a choice of going completely crazy a few to 20 years after the infection and your nose and face caving in and other deformities, or some likely mercury effect. Maybe it worked? Silver nitrate in the eyes of newborns prevents severe conjunctivitis and potential blindness. It used ti be part of a baby delivery first aid kit. Don’t know about today.
Yes mercury was used to treat syphilis in many ways, there was even a joke about the treatment, “A night with Venus, a lifetime with Mercury.”
Our dental clinics have amalgam separators in the drainage system from the dental chairs. To catch it before the waste water is disposed of.
I’ve not had much to do with them but it appears they are like a “cyclonic “ vacuum the heavy particles drop to the bottom.
Very pleased to see scientifically accurate coverage of the phony scares around vaccinations. Thank you Maya! Vaccinate your children and yourselves, everyone.
+ 1 – I agree completely.
The biggest lawsuit in Earth history will be when the dental industry finally caves in over amalgam use in people. According to the US EPA the only safe place for mercury is in a persons mouth. You can’t have your own extracted teeth if they contain that poison. Hazmat! The dentists keep accurate records, lawyers will have a field day. Reparations will go back to the 1800’s.
German; quaksalber-quicksilver. Doctors were poisoning patients instead of using plant based medics and they were called quacks, synonym for incompetent medical care to this day. The Bible was the PDR of that time. Page 1, verse 11 and repeated 19. And the seed bearing plants are for healing.
Tattoo ink.Yikes! Ain’t I glad…
Except its a mercury amalgam. Not elemental mercury (which is much less harmful than its organic compounds). Given how many people have mercury amalgam fillings, and how long they have been used, surely we would see the effects by now? Do you honestly believe that is some grand conspiracy that have somehow been kept secret? Really?
Conspiracy types can not see statistics even when there are billions of test cases over a 100 year span. Applies to amalgam fillings and vaccination (and Roundup eventually).
– Not picking sides on this one, but remember ‘cigarettes do not cause health problems’? and massively less money on the table in that case… Sometimes statistics say whatever someone wants, doctored or not.
I agree withyour point. But for tobacco everyone knew smoking caused cancer and other problems for 200 years or more. The amazing thing is that most just didn’t care. Old time radio has ads for Camels (a really strong filterless cigarette – like Pal Mal and Lucky Strike) with studies that show no throat irritation from pack after pack. In fact even then the tobacco ads were aimed at taking smokers away from competitors. The problem was two-fold. First there was no way to prove a causal link without modern cell biology and the chromosome, meaning 1960’s. Second, aside from the addictive nature for many, the trade-off of appetite suppression and the incredible increase in attention and focus (like 10 point IQ increase) was worth it. I quit smoking in the middle of a project in Silicon Valley in the 1980’s. Quitting was easy. Feeling dumb as a post for a year was not fun.
I would also have to say that is irrelevant to this discussion. First of all the tobacco industry was was huge and and a lot more money was on the table. Also that was decades ago when we knew a lot less. Today our tests are really very accurate and our knowledge of biology is much greater. Wide spread effects have not been seen so the risks must be very low if even measurable.
Please… you make it sound like the dental industry, if all industries, has some sinister control over the world’s governments and it’s only their lack of “caving” that has prevented accountability. I hope you’re not holding your breath on that law suit. Last I checked, cigarettes and alcohol are still for sale, and they’ve killed vastly more people than amalgam tooth fillings.
But go ahead and continue quoting your bible. If you’re looking for a source of death and destruction throughout history… absolutely nothing tops religion.
Not really. And being specific helps. World populations were not even big enough to compete with the count from Communism. But those Quaker death squads were legendary.
You know, that some of the most potent and deadly poisons also came directly from nature, some from plants?
And the traditional Indian (ayurvedic) “medicine” also uses mercury for various things. A few years ago there was a case in Carinthia, Austria where a massive mercury contamination was found at a local sewage treatment facility. So massive that at first an industrial toxic waste incident was expected. It turned out, that a woman, believing in “alternative medicine” and Ayurveda was using ayurvedic “medicine” or cosmetics, she brought with her from india – a few kilograms, containing nearly 2kg of mercury. Probably mostly the red oxide, which is comparatively “non-toxic”, otherwise she would probably have already died of Hg poisoning at the time of detection.
I think she was not punished, because she did not even know, that her “medicine” was that toxic, but perhaps she had to pay partly for damage and clean up.
Sorry to nit pick :)
“Yet for thousands of years, from the Mayans (Temple of the Feathered Serpent in Teotihuacan) to the … “.
Teotihuacan was an Aztec, not Mayan city :)
Organic mercury is not only highly toxic, but passes easily through latex and the skin. Rest in Peace Professor Wetterhahn.
https://sites.dartmouth.edu/dujs/2008/05/16/remembering-karen-wetterhahn/
A professor of toxic metals? I’m just a retired hazmat guy, but we have known for decades that latex gloves are NOT protective equipment. Sad that the professor selected PPE that was improper for the hazards involved. More sad is that such a teaching moment is lost, even trained professor types make errors, we all need to be careful in PPE selection.
Another usage of mercury – the Quicksilver maze game from 1978. That thing was so cool, wish I knew what happened to mine, chances are that it ended up in a landfill. https://www.youtube.com/watch?v=a0PGRWXJ_kg
There used to be a concise list somewhere online maybe 10 years ago plus that listed all the radio-isotopes and I want to say heavy metals also and their target organs and adverse health affects.
Anyone aware of the website I’m thinking of?
Seems like my conspiracy theory was that the information was removed from the public view so those aware wouldn’t be able to identify poisoning methods and poisoning incidents can be compounded and concealed. Personally, I feel there are far more incidents of poisoning going on than most realize as my experience with the attorney and health care professional licensed degree’d professions has demonstrated more fraudulent and malicious intent than I expected. This has been further solidified with the U.S. Diplomat, Staff, Family and others in Cuba, China and elsewhere more recently reported more accurately by CBS News 60 Minutes in their Sept. 1st 2019 report.
Technically, I was trained in my biomedical ethics course that the M.D. Researchers are the most fraudulent researchers in the World.
I assume the J.D.’s are the next most fraudulent and both working hand in hand would compound and conceal the most also to further their interests and concerns to profiteer.
Anyhow, there are well known radio-isotopic tracing methods used for metabolic studies and contrasting for diagnostics and I clearly recall a website that listed the target organs and maybe more detail as well as the adverse health affects with maybe dose threshold info also. Would be a handy chart. Kind of a toxicology chart with practical hazardous applications info included.
I know a guy who bought a government surplus jig bore, about 20 feet tall. A jig bore is a super high precision milling machine, intended mainly for boring straight holes. Problem is he hasn’t been able to use it because of its high voltage DC motors. It was equipped with mercury rectifier tubes and the EPA wouldn’t allow them to be sold with the machine. Nevermind those rectifiers were built to be super strong to ensure the mercury stayed inside them. Nope, they have *gasp* mercury in them so they’re *hazardous*.
Up in Canada they have some long distance high voltage DC transmission lines, with the AC input rectified to DC with massive mercury rectifiers. AFAIK they’ve been in operation for decades without a leak.
As long as it stays in the glass…
I have a few mercury switches in old robots/toys because they’re awesome. I won’t hand them to my son until he gets past the “breaking stuff” phase, but more because of their rarity than health concerns.
I assume the mercury would be inside an insulating container, with metal contacts through it, inside a metal housing.
For sure, probably glass. But glass can be fragile, so “handle with care”.
Why couldn’t the mercury rectifiers in the jig bore be replaced with modern silicon rectifiers?
I suppose they could, if there are ones able to handle the power needs of the motors. Probably would cost more than the surplus/scrap cost of the jig bore.
That machine and a couple of others were set in place on a large concrete slab, then the building was put up. He has a very large metal planer, a Kearney & Trecker 2D mill, various lathes and other mills, a couple of smaller jig bores and more. Most of it’s non-operational and there’s *stuff* piled everywhere, including an antique printing press. He only uses the K&T, a couple of the lathes and drill presses. He built a big X/Y gantry crane and a pivoting gantry crane (which swings over the huge jig bore’s table) but he’s never connected power to them.
When he kicks the bucket I expect the whole lot will likely go for scrap, and actually get scrapped. :( Unfortunately his shop is 300 miles from me. I’d love to own all that stuff, setup so it can all be used, but buying it all, moving it, installing it… He did it starting way back when this “old iron” was practically (or actually) being given away.
In former days also the supply stations for the tramway (city street railway) used Hg rectifier – now the use SCRs. These days we have powerful semiconductor rectifiers and control elements, so there is really no reason to use tubes in that application, let alone Hg containing ones. “High voltage” DC motors – for sure much less than 1kV, as a DC motor with it’s commutator is not easy to build for higher voltage. I think the highest DC motor voltage is about 3kV in some railway systems and if you have a free choice of operating voltage you would prefer a few 100V.
So it would be easy to grab bunch of silicon diodes and refit the machine. Although of course it would be nice nit to be forced to that by stupid bureaucrats. “Greenies” and environmentalists are the worst, they thick they have the license for dictatorship and many people accept this, if the dictator only has a “green” cloak.
No mention of mercury not being allowed on airplanes due to the effects it has on aluminum. On Twitter people were talking about how drones could be used to disrupt air travel, most were dropping bombs or flying into aircraft. A drone spraying planes with mercury would probably be more of a disaster than any other attack method. Many planes could be sprayed and depending how fast the response was the planea might be a total write off! Please don’t anyone do that, its not cool and you’d be a mass murderer! A boiler tech was going to give me 20 oz of mercury, I was like I don’t even know what I’d do with it. Kind of wish I took it because who knows who he gave it to….
Mercury is also used in porosimetry.
https://www.mri.psu.edu/materials-characterization-lab/characterization-techniques/mercury-porosimetry
Quoting a frequency ~6.834 GHz for a rubidium standard and 40.5 GHz for this new mercury-ion standard, to technical people just seems wrong to me. Below are the numbers I would have used.
caesium-133 hyperfine transition working frequency of 9 192 631 770 Hz
rubidium-87 hyperfine transition working frequency of 6 834 682 610.904324 Hz
hydrogen-1 hyperfine transition working frequency of 1 420 405 751.7667 Hz
mercury-199 hyperfine transition working frequency of 40 507 347 996.84159(41) Hz
So the given numbers are a good approximation. As long as you do not want to build such a device yourself, it is not necessary to know the frequency to the last digit.
Since Mercury is heavy easy to move and magnetic in a rail gun it would be most deadly.