In many parts of the world the COVID-19 pandemic is causing shortages in hospital space, staff, medical supplies, and equipment. Severe cases may require breathing support, but there are only so many ventilators available. With that in mind, MIT is working on FDA approval of an emergency ventilator system (E-Vent). They have submitted the design to the FDA for fast track review. The project is open source, so once they have approval the team will release all the data needed to replicate it.
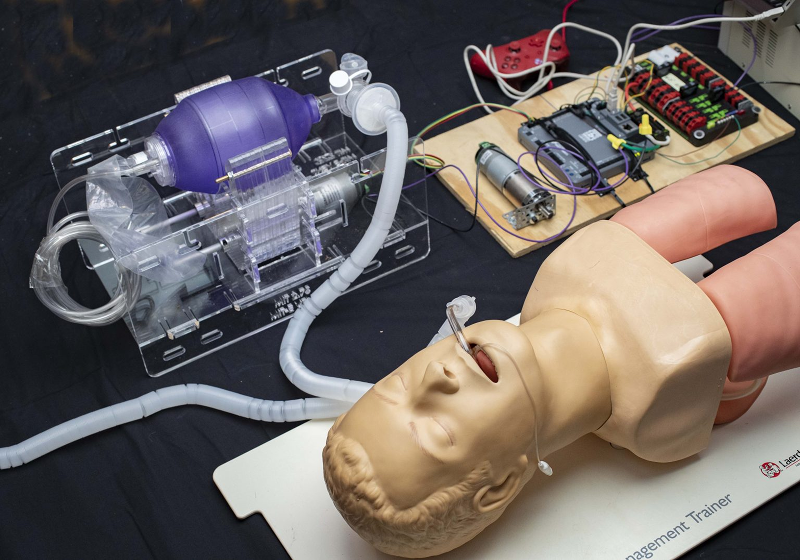
The design is actually made simple by using something that is very common: a manual resuscitator. You have doubtlessly seen these on your favorite medical show. It is the bag someone squeezes while the main character struggles valiantly to save their patient. Of course, having someone sit and squeeze the bag for days on end for thousands of people isn’t very practical and that’s where they’ve included an Arduino-controlled motor to automate the process.
The tricky thing is that, forcing air into your lungs isn’t always good for them. Even healthy lungs can be stressed by too much inflation and people who already have lung problems may be able to handle only a tenth of what a healthy set can manage. That’s why the device needs a closed loop control system that monitors pressure from the patient and modifies the flow.
Any solution should be utilized only in a healthcare setting with direct monitoring by a clinical professional. While it cannot replace an FDA-approved ICU ventilator, in terms of functionality, flexibility, and clinical efficacy, the MIT E-Vent is anticipated to have utility in helping free up existing supply or in life-or-death situations when there is no other option.
Further, any low-cost ventilator system must take great care regarding providing clinicians with the ability to closely control and monitor tidal volume, inspiratory pressure, bpm, and I/E ratio, and be able to provide additional support in the form of PEEP, PIP monitoring, filtration, and adaptation to individual patient parameters. We recognize, and would like to highlight for anyone seeking to manufacture a low-cost emergency ventilator, that failing to properly consider these factors can result in serious long-term injury or death.
This isn’t a unique idea, and the MIT team provides links to other similar projects. The team’s work is not totally online yet, because they are still testing. For example, the acrylic apparatus that squeezes the bag may not hold up to the repetitive stress very well. The team may look to other projects that predated the crisis. For example, have a look at the AIR device presented at a conference last year in the video below. There’s also this interesting document from a Johns Hopkins resident.
Almost as interesting as the device itself is the comments people are leaving about the design. It is a great example of how the Internet opens up totally new ways to collaborate on a critical problem like this one.
Of course, we’ve seen collaboration on COVID-19 testing, too. If you want to help, you can add your compute power to the virtual supercomputer folding proteins to help find a cure.















Well in these times improvisation is what we need, and to quote the hackers favourite movie, Wargames.
https://www.youtube.com/watch?v=6uVunn6Vux4
https://www.gov.uk/government/publications/coronavirus-covid-19-ventilator-supply-specification/rapidly-manufactured-ventilator-system-specification
What we really need is a ventilator that meets these specs. Ventilator is a broad term.
Hopefully this sufficiently meets some of the roles required.
Nice link, sometimes funny to:
(Quote) must connect to wall pipeline oxygen supply via Schrader valve connector (BS 5682, not the bicycle wheel version) (/Quote)
At the very bottom there is an estimation that approximately 30.000 of these things are wished for.
Shameless self promotion time.
https://hackaday.io/project/170507-cosv-cam-open-source-ventilator
Eliminates the tube in the MIT design, which is prone to CO2 buildup.
Don’t know if you’ve ever actually used a BVM for resuscitation. I used to be an EMT and I have actually used them on real patients. Your design will have tremendous difficulty maintaining an air tight seal.
CO2 buildup is not as big of a problem as you expect because during the exhalation phase, air shouldn’t be pushed back up the inlet tube. There is a separate outlet tube shown in the picture and likely there is a check valve at the three-way junction of the inlet tube, endo-tracheal tube, and outlet tube.
A ventilator has zero efficacy if an air tight seal cannot be maintained because the air will not go into the patients lungs unless positive pressure can be maintained.
Yes, their tube is much shorter to the Y junction than most BVM implementations I have seen, but it still exists.
As for maintaining the seal, I have a small plate which attaches between the mask and the valves which very closely mimics the attachment setup of a chemical respirator. I have yet to document that at all. Also not documented is that the stepper is being replaced by a 30k hour rated BLDC gear motor of greater torque and 1/4 the weight on Wed… so the mass of the portion of the design that is on the bag, on the patient’s chest will be minimal. Also not documented is the two pressure differential sensors which will be used to detect any abnormal situation, and a whole lot more. One thing at a time.
Making medical equipment is not some game…. but they do have a Wii gamepad in the posted image..
I’ll see my way out now
It might only be there for demo purposes or as a readily available, low-cost control interface. Of more concern is if any of the parts are sole sourced from China or otherwise non-domestic sources that may delay implementation.
A user interface is a user interface, regardless of it’s source. If it does the job, is inexpensive, readily available in quantity and has acceptable reliability, it makes no difference what it’s original purpose was.
Actually the UI does make a difference in a medical environment.
can you elaborate on this?
In there… https://www.gov.uk/government/publications/coronavirus-covid-19-ventilator-supply-specification/rapidly-manufactured-ventilator-system-specification
I took a quick pass through the UK document and did not see anything that could not be met by a handheld game for a user interface. It seems like the qualifier:
“If it does the job, is inexpensive, readily available in quantity and has acceptable reliability” covers it. Everything else is software.
The controller may be in the picture, but it does not appear to have any relation to the actual electronic controls of the machine…
https://e-vent.mit.edu/controls/
A game controller is a very convenient USB or Blue-tooth based source of HID input, so it may well be very practical in this case.
“Manual Ventilators” are usually called “Bag Valve Mask” or “Ambu Bag” in the medical world.
This looks like a really great idea. Ambu bags are simple and most hospitals have lots of them in stock already. They come in Adult, Pediatric, and Infant sizes (and most hospitals stock plenty of all three). The apparatus looks like its made mostly of simple laser cut acrylic and a stepper or servo motor to actuate it under control of a micro controller. I’m guessing that the game pad allows doctors to adjust tidal volumes, cycle rate, etc. Genius, actually!
GTR IV (Grand Theft Respirator)
Illustrates what I have always felt about engineers: Smart; Creative; Practical.
Disclosure – my Dad was an ME, and he was all of the above
As to the device, based on my years in the ICU this is far more sensible than a Tee connector to one Vent and two patients – That is truly an act of desperation.
Here’s another from the University of Minnesota.
https://coventors.com/
I checked it too. Is pure crap. They should open the schematics and the desing to the world so we can colaborate. In my opinion is an absurd to start something like that, say that is for the good of the Corona people, and later dont share anything.
https://oxvent.org/ another from Oxford UK
I checked Oxvent. I pure crap. They should open the schematics and the desing to the world so we can colaborate. In my opinion is an absurd to start something like that, say that is for the good of the Corona people, and later dont share anything.
Good job guys, I’ve been working on a few concepts along this line and trying to find the show stoppers for getting these systems out. A few questions:
1) I couldn’t tell if there are rollers in contact with the bag, I’d assume so. Is so, try to match the compliance of the roller surface to match the bag material to balance the eventual abrasion and wear over 760,000 potential breathing cycles.
2) The re-usable bags are silicon and can be sterilized. Yes, they cost a bit more, but should be able to withstand the cycles better. My assumption, at this point, as I haven’t done the life cycle testing yet.
3) I’m assuming that the compliance of the bag will change locally and increase the likelihood of failures. It looks like your design allows for bag rotation… how often and how much should be decided.
4) It looks like the structural elements can be laser cut/cnc cut acrylic. When you open source the solution, how do you see the local maker communities contributing? Do you have guidance on integration and testing before deployment? HardwareX or Github/lab?
Congrats on getting it in front of the FDA, will you be able to share their comments with the rest of the community?
Ventilator: couldn’t you use a CPAP ?
Seems like a better Hack, Since most of the people that have respiratory problems have CPAPs at home.
(and they have pressure feedback, O2 injection, valves, etc.)
As I understand it, CPAP machines aid in transmission of the virus by pushing aerosolized particals into the air around the patient, making it a dangerous environment for health care workers.
I wonder if this particular issue could be resolved with some adjustments to the plumbing. CPAP plumbing is all modular, though I’m not aware whether or not they are compatible with the hoses used in ventilators.
Also, there are microbial filters already readily available on the market for CPAP machines. I also wonder if those could be adapted to aid in this issue.
Adapting CPAP devices to the COVID-19 situation is probably the fastest, most efficient way to create a severe shortage of CPAP devices.
” Last year, the AMBU® Bag concept was re-visited by two student teams, one from Rice university (here & here), and another Boston-based team who won MIT Sloan’s Healthcare prize (MIT News: Umbilizer).” from the MIT website.
Umbulizer has a patent application http://www.freepatentsonline.com/y2019/0336713.html so could someone tell me what part of their design they are trying to protect? The general mechanical concept would seem to have prior art from the 2010 paper (http://web.mit.edu/2.75/).
Seemed they were trying to claim priority over a 2018 application, so could have been anti-troll measures.
Arrrgh Dudes and dudettes! Quick question(s)…How many of you have actually kept a patient alive and breathing with an Ambu bag? On a helicopter on the way to a hospital? On an ambulance on the way to the hospital? In a hospital ICU waiting for a proper Vent to be brought to the bedside? How many have used a manual resuscitator by any brand in an ER for an extended period of time?
These bags work. Plain and simple.
The design works, it makes sense. So…. how fast can these be made as soon as the FDA puts it’s seal on them? Can we as a hacker community stand up and supply the parts (if even needed).
The world awaits.
Ambu bags are life-savers and every hospital already has a supply of the bags on hand that far outnumber vents. Not to say that supply chain is not a factor, but at least the a critical component is available at the outset. First thing I did when prepping an ICU bed for an incoming patient was confirm I had a bag of appropriate size at the head of the bed.
Just did a google search and came up with this
Flow Restricted Oxygen Powered Ventilation Device
https://slideplayer.com/slide/5048937/16/images/53/Flow-Restricted%2C+Oxygen-Powered+Ventilation+Device.jpg
could these be used in place of ventilators, could they be used on an intubated paitent?
Sourcing the components once the design is available is key…do we have a probable list ?
This. Almost anyone that looked into home brew respirators has come up with some variant of a servo pushing the bag with some quickly built arms.
Most focus on electronics and software, but please do not forget that these devices should last a few million cycles. So design the bag-pushing mechanics in a more durable way, because some thin sheet of laser cut acrylic or 3d printed PLA might not withstand more than a few days at 12-18 breaths/minute.
And as an addendum to think about, as we have seen in the last few weeks, supply chains are not as robust as we used to think. Things as common as cheap masks or as mundane as isopropyl alcohol or ethanol apparently get almost impossible to source short term right now in many places of the world.
It is critical to design around components that can be sourced in really large quantities and result in a reliable devices. Or can be adapted according to local sourcing capabilities.
Don’t forget a UPS! Vents have built-in backup batteries. And, most hospital rooms have UPS-sourced outlets. However, in the field hospitals that are likey to be constructed, short term power would be a critical component too.
Bags last a very long time. The good news is the bag is already hooked up so you can hand bag while a new mechanical or control aparatus is swapped out.
Suggestion: Replace the Arduino with something far more robust such as a decent quality PLC, if available of course. (sometimes ya just hafta run whatcha brung)
1. Having experience with FDA validation, this would require a good amount of time to validate if software is used. to get it approved quicker use relay logic, timers. can pass validation very quickly. Could do this simultaneous with Arduino or plc version. The non software version will finish testing and approvals much faster. In reality these can be simple devices.
2. Nothing wrong with Arduino, could also just use a PIC and custom circuit board produced locally. Should be no problem to source components as there are many options. Again could help with validation as software of write once pic is protected.
3. From my hobby experience with plexiglass parts, I would suggest using aluminum or other metals on parts for longevity where needed. I know some machinists (local) that know fully well how to mass produce parts efficiently with very high precision and would think there are plenty nationwide that would be willing to do their part.
The FDA is the difficult part of equation, they most likely will not want to wave validation requirements. Get experience professional help onboard it will save a lot of time.
of course these comments are based on the US. for people in other locations around the world, I’ll bet many would be able to use the device as it is currently shown.
Thank you to everyone involved.
I’m not sure, since 21CFR820 is federal regulations, might the president be able to waive requirements until validated equipment manufacture can get up to speed? All I ever did was lab equipment and part 11 work, and not for long. I know its a huge pain in the ass. My buddy runs live specimen tests for facility validation of SIP systems and they just keep running them until they pass–he left that job because of it. Its crazy how things actually happen in the real world.
The base frame, arms and parts could be injection molded in self lubricating acetal which would last much longer than fabricated acrylic which wears fast and tends to crack. Aluminum molds can be built here in the USA in 2-3 weeks. Yes, I work in injection molding and we can do this.
So weird how people can think the same solution thousands of kilometers away. Yesterday i came across this video on facebook that uses the same bag.
https://www.facebook.com/1133134209/posts/10220265788051533/?sfnsn=scwspwa&d=w&vh=i&extid=u1kTnBWzemz1OQQE&d=w&vh=i
The motor/gearbox combo shown here is sourced from China and is unlikely to last long. It seems underpowered for the task. How about using a drill – its motor might be same, but gearbox is usually more robust. DC or AC variety. Plenty in stock in the US, I’d guess.
I also wondered about making a drill (perhaps with AC adapter) turn a cam, which cyclically compresses and retracts a parallelogram to squeeze the bag. These could be made with hardware available the world over, without reliance on a national or international supply chain. Not sure if the drill motor driver will be good with the trigger partially pressed for hours/days at a time.
Doubt the pressure sensors are reliable/robust at these low pressures 20cm/h2o. Have used some on ventilation systems and they are easily damaged/changed by over pressure, e.g patient coughing.
What’s wrong with pressure limitation via a literal water column?
Absolutely nothing. Water seals have been used on Chest Drain systems for more than 150 years.
“The use of chest tubes was described as long ago as the time of Hippocrates (c. 460 BCE), when metal tubes were placed to treat empyema. Playfair, treating a child with empyema thoracis in 1873, is credited with being the first physician to use a water-sealed chest drainage system.”
https://journals.sagepub.com/doi/abs/10.1177/1553350619868369
Hi, am doctor turned Biomedical Engineer. We have used these bags for days together to treat rat poison cases. The patients close relatives would do the pumping in turns. There were only 2 ventilators and sometimes 5 patients would be on ambu bags. Patients were conscious and not paralysed (their respiratory arrest was due to respiratory muscle failure) and we had a good survival rate. This was in 1995. Not sure and will not be surprised if the same is the case today too. Students like me from Stanley Medical College, India can boost that we can manage any patient emergency.
Now as a biomedical engineer, I would suggest that going the BiPAP way would be right thing for this emergency. FDA has recommended that BiPAP may be used for respiratory support. As alveolar damage progresses and Respiratory Distress sets in, the BiPAP will take away the ‘work of breathing’ from the patient’s respiratory muscles and prevent him from going into respiratory failure due to exhaustion. BiPAP in my opinion should be first line of respiratory support. If pSO2 further diminishes 100% oxygen can be fed into the BiPAP. Further detoriation can only be saved by a Heart Lung machine. I recollect the mortality rate on patient on Ventilators is 84% in Whuan. But something has to be done on the gasping patient. In Stanley we have never turned away a patient to certain death. Until the last movement we give CPR. Patients being turned back in Italy due to lack beds is saddening to me. Can say now, but at least in normal times we would have put them on the floor, given an ambu bag and made the patients relative to pump.
Design of BiPAP is a lot easier. A hack to turn a CPAP to a BiPAP can also meet the emergency. Well CPAP itself can take away the work of breathing to some extent. And the good thing is there are millions of these BiPAPs and CPAP’s in the USA unlike in other countries.
Unfortnately Ambubags, or these manual resculators are used in many countries as ventilators.
My wife, from the Phillipines had to take turns with her relatives pumping the bag to try and keep her grandmother alive until an electrical ventilator became available. High cost electrical ventilators with all the bells and whistle are rare in less fortunate nations. There is actually hundreds of these designs to use manual resculators as ventilators.
The simple fact, there is not enough electrical ventilators in the world for the current need.
These Ambubag ventilators can be used as back-up devices when no “Marketed Ventilator” is available. Which will probably happen very soon in some areas.
Anything and everything needs to be brought to the table.
Ambubags work, they are cheap, between 10 and 20 dollars and they just need a pumping device.
100’s of thosuands currently exist all over the country in every fire station and ambulance service.
It should be used as a back-up device when nothing else is available and hopefully it will keep people alive.
Another possible approach is to wrap a blood pressure cuff around the the Ambubag ventilator, connect to a portable air pump (aquarium air pump) thru a battery powered air valve and controlled by a off-on timing circuit.