To say that a lot has happened in the year since the COVID-19 pandemic started is an understatement of epic proportions, so much so that it may be hard to remember how the hardware hacking community responded during those early days, with mass-produced PPE, homebrew ventilators and the like. But we don’t recall seeing too many attempts to build something like this DIY oxygen concentrator during that initial build-out phase.
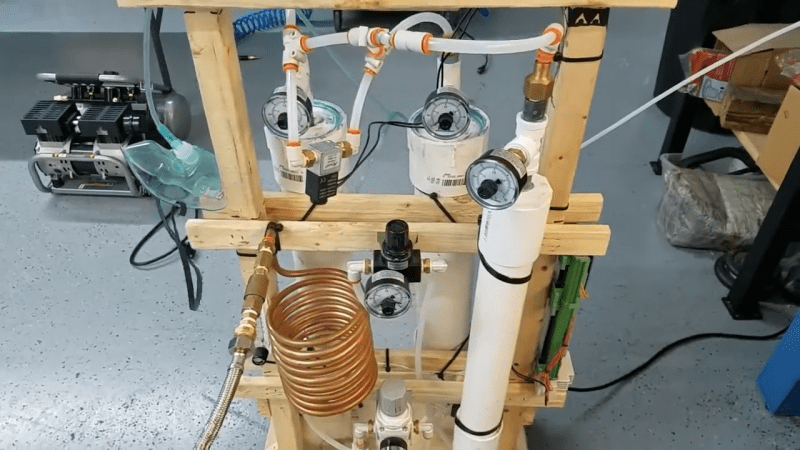
Given the simplicity and efficacy of the design, dubbed OxiKit, it seems strange that we didn’t see more of these devices. OxiKit uses zeolite, a porous mineral that can be used as a molecular sieve. The tiny beads are packed into columns made from hardware store PVC pipes and fittings and connected to an oil-less air compressor through some solenoid-controlled pneumatic valves. After being cooled in a coil of copper pipe, the compressed air is forced through one zeolite column, which preferentially retains the nitrogen while letting the oxygen pass through. The oxygen stream is split, with part going into a buffer tank and part going into the outlet of the second zeolite column, where it forces the adsorbed nitrogen to be released. An Arduino controls the valves that alternate the gas flow back and forth, resulting in 15 liters per minute of 96% pure oxygen.
OxiKit isn’t optimized as a commercial oxygen concentrator is, so it’s not particularly quiet. But it’s a heck of a lot cheaper than a commercial unit, and an easy build for most hackers. OxiKit’s designs are all open source, but they do sell kits and some of the harder-to-source parts and supplies, like the zeolite. We’d be tempted to build something like this just because the technology is so neat; having a source of high-flow oxygen available isn’t a bad idea, either.















15 Liters a minute seems very impressive. For scale, that’s enough to keep 7 people a live (@ ~2 liters per person per minute) in nominal circumstances.
I always wondered how these could work. Very interesting. It almost seemed to violate the laws of thermodynamics, but it doesn’t.
With that much oxygen production, I wonder what would happen if you hooked this baby up to a car engine, and/or scaled it up. It might be like nitrous. It would be fairly safe because you could set it up so that “pure” oxygen produced is consumed immediately very close to the engine and not stored anywhere. I’d tune the car up first, though. Backfires…”would be bad”.
I think it would be good for oxy/propane , oxy/hydrogen, or oxy/acetylene welding / brazing / cutting.
True, after I watched this video YT popped up a suggested video by Dalibor Farny on an O2 concentrator he built to supply the oxy-fuel torches he needs on his glassblowing lathes for building his custom nixie tubes. It’s essentially six of these ganged together to produce 30 lpm O2.
https://www.youtube.com/watch?v=xt_ey-DTEOw
Id help dig up 2,000lbs of potatoes For one of those Nixie Clocks!
I guess a 2 liter engine running at a few thousand RPMs might consume those 15 liters rather more quickly than 1 minute. But maybe it could raise the oxygen level in the intake air enough to be significant? No idea really.
2 Liters full throttle 4 stroke 4000 rpm = 2 * 4000 / 4 = 2000 Liters per minute.
(2 ltr x 4000 rpm)/2. (4 cycles INTAKE every other revolution.) General use/normal operation, (cruise;) maybe 3000 rpm for a small 2 ltr engine. (2&3000)/2 = 3000 lpm.
SUPPOSE you enrichen the 20% O2 by 10%… (3000 ltr x 0.2)/2 = 300 lpm O2 normally used. 0.1 * 300 = 30 lpm O2 addition. 3 ltr/min would be a 1% addition and may be helpful…! Esp at elevation.
Nitrous gives power as much because it releases a nitrogen molecule for every nitrous oxide molecule broken down (maintaining its volume as the oxygen is consumed) as it does for raising the effective oxygen concentration (the release is also exothermic). Pumping in pure oxygen isn’t as beneficial because you still lose volume and have to contend with potentially igniting the engine block.
Also, an engine is using thousands of litres per minute .
This… does not seem optimal…
Exciting though.
Engine rich exhaust
you would need a serious scale-up. A car engine of 2 liters rotating at 2500 rpm “breathes” about 2.5 cubic meter of air (21% O²) per minute. About 600 times more than a human at rest.
A human consumes about 25% of the O² it breathes, but a car about 90%….
Also burn really hot and melt pistons. You can actually get heaps more power from any engine by leaning the fuel mix. But the piston tends to melt from the increased heat. Lower oxygen levels stop metal melting.
Common automobile engines are airflow limited and produce maximum power when all the oxygen in the air is burned. This is achieved for a slightly rich mixture, which leaves a little gasoline unburned. Car engines are usually run slightly lean except when maximum power is needed, because running rich means poor fuel economy and increased hydrocarbon pollution.
Necro-post I know, but this is definitely incorrect. I tune/re-tune ECUs regularly and engines are by default running slightly richer than stoich. The reason is for, you guessed it, reduced emissions. Consumer ECUs and catalytic converters are designed around this slightly rich tune.
Its a fairly trivial matter to re-tune an engine to be stoich and net a rather small fuel savings, this of course increases tested emissions.
Of course, one can tune an engine even leaner for the power gains, (and again fuel savings) but the farther you go the more fiddly the tune gets and eventually you require higher octane fuel.
A good WBO2/dyno tune isn’t cheap and the most basic of these tunes starts at $500. Tuning for economy AND across engine loads and gear selection is where things start to cost more and more (typically more than you will net in fuel savings) and outside of saying you can/competing, it really isn’t worth it.
If you want to use this to increase power, you’d need a way to trick the engine computer into adding a proportional amount of fuel at the same time.
If you can keep the air-fuel ratio constant, then it’s roughly similar to just opening the throttle by an extra few percent.
But if you go much beyond “a few percent” (deliberately vague…) you might reach the limit of the ECU’s ability to understand how much air is coming in, or control how much fuel is going out, or set the right spark timing for whatever RPM and airflow you’re at.
Danger to Manifold!!
Or I wonder if the nitrogen could be fed into a plant growing system…that would be mind blowing…
Flow rate required to keep someone alive very much depends on how sick they are! 2 l/min is quite a simplification. Many sick patients on intensive care would need 15 l/min.
Just be careful what you run that oxygen over. High-concentration O2 makes many things flammable, and drives many oils and lubricants to self-ignite. That’s why they use an oil-less compressor.
That plus nobody wants to inhale oily oxygen…
Oxygen bubbled through water.
Or fire 😜
That, and the many other “not immediately intuitive” ways mis-handling O2 will hurt you, especially as the pressure get’s higher.
If you’re playing O2, Vance Harlow’s Oxygen Hacker’s Companion (Nitrox diver may own this already) is a handy text to have around:
http://www.airspeedpress.com/newoxyhacker.html
I had no knowledge of that book, being a user and not a mixer. Still, thanks for the reference, I’m ordering a copy as soon as the form works!
And the “PVC Pressure Vessel” doomsday comments start in 3…2…1…
Yep, I’ll mention it. PVC’s failure mode for compressed air is an explosion with shrapnel, so watch those pressure ratings carefully – they get lower as pipe diameter increases.
In the early 1980’s, I worked for a medical equipment leasing company that both leased and serviced Devilbiss oxygen concentrators. At the time, those units were the size of a small beer fridge. I distinctly remember the “hardware store” nature of their internal construction. I also recall the sieve beds as having been fabricated from 4″ PVC tube and caps, so the construction depicted in this project is consistent with prior historical (but apparently practical) art.
The compressor was a double rocking-piston/diaphragm type, which kept the compressed air oil-free. The valves in the compressor heads were thin stainless steel reeds.
Flow sequencing was done with a mechanical timer–no Arduino needed. The timer had a synchronous (clock-type gear motor) that drove a shaft with multiple cam wheels. Micro switches riding the cams fired solenoids, moving the gasses around.
Back then, those machines were capable of 98% O2 at better than 2 LPM.
The biggest enemy of these machines was high humidity. Adsorption of water molecules ruined the sieve beds.
Just before I left the company, we started acquiring concentrator units from a Devilbiss’ competitor (whose name now escapes me) that already showed significant improvement. In addition to the newer concentrator being physically smaller and quieter, the company built their sieve beds from aluminum tube. The tubes were capped with plates featuring machined grooves for O-rings. I seem to recall all-thread struts holding the assembly together. The nice thing about that design is that the beds could be taken apart and the sieve material replaced, if necessary. They also dispensed with the mechanical timer and replaced it with simple electronics and SSRs to fire their solenoids.
Very informative & interesting. Thanks👍🏻👌🏻
I love the simplicity of the mechanically timed valve switching. Do you happen to have any old schematics or pictures that you could legally share? Eliminating electronics where practical has advantages. I read that solar flare activity is likely to increase to levels that will wreak havoc on electronics and little things like the grid.
They do call out to use SCH40 pipe (rated 260psi @ 3″) and explicitly have a 40psi relief valve and 20-30psi regulator ahead of the pressurized PVC so there’s a decent safety factor there. Not sure how exposure to O2 will change that strength though.
But the pipe in the video quite clearly says on the side “not for pressure”…
SCH40 burst pressure is many times higher than the rated pressure – depending on diameter. That 3″ pipe is around 850psi and 6 inch is around 500psi. 1/2 inch is nearly 2000psi. Double the numbers for SCH80. This is why the PVC tennis ball launchers don’t blow up – much. Scaling them up to 6 or 8 inch combustion chambers is pushing your luck. But in general the hacker community tends to grossly underestimate the strength of the plastic piles. https://www.pvcfittingsonline.com/resource-center/strength-of-pvc-pipe-with-strength-chart/
I’d be interested in dialing down the capacity (and probably purity) to use for hobbyist flamework. The hobby market typically buys retired medical oxycons. That was my first thought, but the kit cost + BOM is way over the price of a retired medical unit.
A 2 liter automobile engine can consume 9000 liters/minute of oxygen (high revs), so 15 liters/minute is about 600 times short.. I’m not sure you would actually notice the boost.. But as a life-saving apparatus, it’s a cool device. I picked up a couple of 5 liter/min refurbished concentrators for about $300 each (the prices seem to be on the upswing). Creating 5 liters/min. uses a couple of hundred watts, so extrapolating to 9000 liters/min (just for fun) would take about 360kW (480 hp).
Why is Norton flagging the Oxykit link as “Dangerous Website” ?
Why are people still using Norton … in 2021 ???
Because their algorithm was written by the band Berlin. (Figure that one out and you get a gold star.)
Because it takes their breath away.
Well I was talking to “bkwilli” but you still get a silver star. *
Install teh Linux and the pain will stop.
I went looking for “zeolite”… The devil is in the details.
The question then became WHICH of the many zeolites are in use here. Hmmm…
Looking at the company web site… Well, the specification in their store is a bit nebulous but they’ll sell you 5 pounds for $75.00 US. So let’s go look at github. Nope. Nothing in any of the BOMs there either.
We have an opensource electro-mechanical design that tells you how to build it but not how to fill it. I’d call that a crucial bit of information missing. As a certain character would say with a raised eyebrow… Fascinating.
OxiKit mentions in the comments to one of their videos — the one I linked to in the story, IIRC — that this is sodium zeolite.
Per wikipedia, There are a number of sodium zeolites Dan.
Just like any other molecular sieve – you tell the manufacturer what you want to use it for, not what it should be made of. Because they’re all the same stuff, just with different pore sizes.
13X zeolite 0.4 mm – 0.8 mm or JLOX 101 are both commonly used in o2 concentrators the second one is the most expensive. I used the 13X when rebuilding my craigslist o2 concentrator. The green light is on so the o2 purity is at least 94 percent.
Is it possible to “regenerate” the zeolite if it’s moisture contaminated. My thought is slowly heat the zeolite in an electric oven. so as to not damage the pores as moisture leaves it, while avoiding generating water vapor and carbon contaminates from burning either methane or LP.
The most common molecular sieve for oxygen/nitrogen concentration is 13X. (10 angstrom pore size)
https://catalysts.basf.com/files/literature-library/BASF_13X-Molecular-Sieve_Datasheet_Rev.08-2020.pdf
5A (5 angstrom pore) molecular sieve can also be used. I think it’s a bit less selective for nitrogen but still usable.
Very interesting technology, never heard of PSA (Pressure swing adsorption) before.
There is good animation on wikipedia that visually helps to understand how the device works:
https://upload.wikimedia.org/wikipedia/commons/7/76/Pressure_swing_adsorption_principle.svg
I compressed air input
A adsorption
O oxygen output
D desorption
E exhaust
When one zeolite column is nearly saturated with nitrogen, the valves are all fliped to release that column’s adsorbed nitrogen.
Thanks a lot for your simple explanation. I’ve always wondered if a nitrogen generator was within a reach of DIY project for nitrogen soldering at home.
So basically the waste output of an oxygen concentrator is nitrogen: perfect, I’ll have it for my lead free soldering station.
Indeed, for the hobbyist, being able to turn air into mostly pure oxygen and mostly pure nitrogen is potentially very useful. I wonder if you could use the “mostly nitrogen” as a shielding gas for welding.
For TIG (aka GTAW), I’m not sure as the plasma plume is very sensitive, argon is mostly used, sometimes with a pinch of helium for penetration in materials such aluminium and titanium. Also flow is about 6 to 8l/min, probably too much for a standard compressor.
For soldering, definitely, major soldering station brand sell nitrogen shielding gas for rohs production but it’s in the 1-2k€ for complete kit. They have a flow of about 1l/min, perfect for molecular sieve.
So let’s rig some hardware and have fluxless lead free soldering at home!
Welders would love being able to use straight nitrogen as a shield gas. It’s way cheaper than argon or, worse, helium. Unfortunately, it’s plenty reactive at the temperatures that an electrical arc reaches, and tends to form undesirable nitrides in the weld.
It IS used in welding shield gas, but only in proportionately small quantities to alter the characteristics of the weld.
Apparently, it’s a viable thing to use in laser welding, but that’s not exactly something even a well-equipped fab shop is likely to have.
By volume, dry air contains 78.09% nitrogen, 20.95% oxygen, 0.93% argon, 0.04% carbon dioxide.
So in theory, at least, you could reduce the nitrogen with one PSA, and then reduce the oxygen with a second PSA (using a different zeolite) and be left with a higher concentration of everything that is not oxygen and not nitrogen.
While you are right Truth, at that point its easier I would suggest to just condense the air and then distil to separate out the gases you want/don’t want..
@Foldi-One folding point in terms of energy in and gas out I fully agree that at a large scale that would be far more efficient because you can use the evaporation for pre-cooling.
But at very small scale you would have the initial cost for 1 compressor, 4 zeolite columns and a bunch of electronic pressure valves and cheap controller (The Brain) would I think be less.
@irox Definitely get the analogy, but no one who is on 2L of oxygen will die/deteriorate rapidly from not getting oxygen. For comparison, our ICU patients who are on high flow secondary to COVID are getting 45-55L at 60-90% FIO2. Those are our “sick-stable” patients, aka will definitely deteriorate quickly without high flow, but not so ill that we would intubate. You’d see similar or higher numbers for other ARDS patients or most other scenarios that require anything greater than regular nasal cannula.
To me, the usage is niche. This could reasonably sustain 2 patients on 6-8 L which is really where high flow shines above just regular nasal cannula or NIPPV. I’d say this would work well for a small hospital who is limited by their oxygen supply and would be able to keep a moderately sick patient supplied in a short term supply emergency.
Wow! Thanks for the additional context.
Are the patients consuming 6L (or 45-55L) of O2 a minute, or is part of that loss, exhaling to the environment or something?
My context/experience is solely limited life support systems for health people (where CO2 is removed and around 2L per person per minute is added) so thanks the medical usage numbers, kind of an eye opener!
Gotta remember that they’re on oxygen because their lungs have got realllllly crappy at taking up oxygen. Hence a high degree of overhead vs what the theoretical needs of the body are, because very little is actually getting into it.
I don’t know if the guy talking is the guy who designed it, but that doesn’t work the way he is describing. Molecular sieves and zeolites do not trap N2, they can trap O2. To trap N2, you need a nitrogen getter, which is a completely different animal. The sieves trap O2 at pressure, while the N2 continues through, and this must be true, because when you release the pressure and use it to dump out the N2 in the other column, it would make no sense to try to remove N2 with N2. This is a pressure swing adsorption unit (PSA), and they work by trapping O2. Better efficiency comes with higher pressure and more cylinders (up to 85% efficient with 4 cylinders). This DOES concentrate O2, but it doesnt work the way he says (or the article says)
You’ll have to come up with a source for your claim, because you absolutely can adsorb N2 onto 13X and 5A zeolite molecular sieves. http://www.phys.ufl.edu/REU/2008/reports/magee.pdf
The Wikipedia PSA article also confirms Zeolite adsorbing the Nitrogen. https://en.wikipedia.org/wiki/Pressure_swing_adsorption#Process
“But it’s a heck of a lot cheaper than a commercial unit”
With a BOM that exceeds $1k, I find it hard to support that statement. Commercial concentrators for home use (non-portable) are easily found for close to 1/3 the BOM cost, and there’s no labor involved. I get that 17LPM is cool, but no one outside of a hospital would ever require such a flow rate. Anyone with such requirements is either close to checking-out or is going to intubated.
Yes this is a cool project but yes the cost effectiveness is somewhat negligible NEW 10l/pm units in Australia are only around $1500AUD Im presuming the $1000 is US dollars which makes it cheaper to buy a new unit.
BUT
if you cant get a commercial unit and you desperately need one this is one way to do it.
I bought one off eBay just before the pandemic for around £160 with a 1.5l/min flow rate at 98%.
And it’s a damn sight quieter than this thing! So you could actually sleep while it works.
But having said that this is an excellent effort. Put it in the next room on a long pipe, to avoid the noise, and explosion risk…
I have 2 5L/min units I got free, thought I was looking for them for a long time.
i wonder if you could potentially use this as a source of almost pure nitrogen for applications in protective atmospheres and maybe even welding?
I’m thinking produce storage. Apples do great under cool nitrogen.
How about nitrogen filled tires. Nitrogen must REALLY be expensive considering what they charge for this service… :)
I think nitrogen filled tires are about 80% racket anyway…
Nitrogen filled tires are not really necessary for car tires, although the nitrogen keeps your tires inflated longer than oxygen does. Nitrogen is required for filling some (most? all?) high pressure aircraft tires (I don’t know the max, but 757 main tires are filled to 205 psi). I can think of two legitimate reasons: 1. Nitrogen from a cylinder is completely dry, where compressed air often has a bunch of water and oil in it, and 2. The oxygen in compressed air oxidizes the rubber (which means it’s no longer available to pressurize the tire), gradually (if the system is at a reasonable temperature) or possibly very rapidly (as after a maximum-effort braking with a rejected takeoff). I still fill my car tires with nitrogen, and the tires that I do that with don’t need a few p.s.i. top-up after a few months, but I wouldn’t pay a tire shop $20 to do it because I can just check my tire pressure again in a month or two.
There could be next very interesting step – get this concentrator output and separate 95% O2 + 5% Ar mix. This could be done using CMS molecular sieves in PSA system using kinetic separation. And then build 150bar pump to fill Argon cylinders. :)
Now we just need someone to do a home implementation of the Linde process for some truly explosive fun
https://youtu.be/eRVKb0R0hwk
oxygen+gasoline= Petrogen cutting torch
oops wrong link
https://youtu.be/SabpLuqd2ZA