[Tim] recently found himself tinkering with a cheap string of LEDs. Far from an advanced, IC-controlled addressable set, these were merely a string with LEDs of four colors that could be switched on and off. However, digging in to the LEDs themselves turned up a curious find.
The LEDs were set up in a parallel/anti-parallel fashion. The two power lines ran the length of the string, with all the LEDs installed across them. If polarity was applied in one direction, the red and yellow LEDs would light up, in the other, the blue and green LEDs would light together.
This raised a question for [Tim], as typically, different LEDs light up at different forward voltages and this can cause issues when running different color LEDs in parallel together. What he instead found was that all the LEDs were actually blue LEDs in their fundamental construction. However, the red, yellow, and green LEDs had all been given a phosphor coating. In these devices, when the blue LED underneath lit up, the phosphor converted the light into the desired color. [Tim] was able to confirm this behaviour by illuminating the phosphor manually using an external UV-A LED.
It’s an interesting choice, but it’s certainly one way of making a multicolored string of LEDs. If you wanna get fancier though, consider studying this tutorial on working with addressable LED strings!
[Thanks to J Peterson for the tip!]

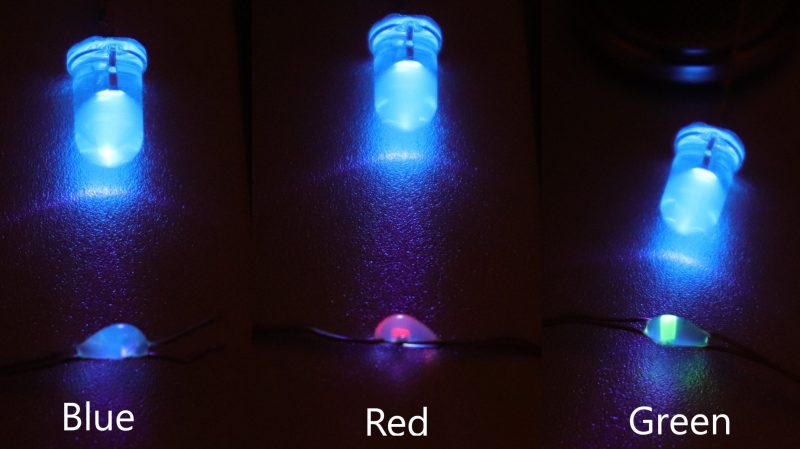














Neat!
A Blue-Pump LED for red, yellow & green, like Blue-Pump white LEDs.
I would be interesting to see if the current is the same for each color:
– are the under laying Blue-Pumps the same for each color and/or
– are they driven the same for each color?
And what are the generated lumens for each color.
I expect the blue-pump approach is to deliberately avoid dealing with differences in driving. Per the post, they’re all just connected across the 2 wires. It’s cheap. It’s easy to assemble.
Im the inventer of much in rgbw tech technologies known as light based technologies.
No 2 leds are the same.
Iv been to china teach factories iv addressed LEDs using multiplex series parallel.
Going back 20 years.
I think the coating does not influence electric behaviour. So yes the current should be the same. But how does the brightness change with the colour?
The blue LED with phosphor coating concept isn’t new. That’s how the “filament LED” bulbs (the ones that try to look like the older incandescent bulbs) have been made for years. They are simply a string of LED chips mounted on a thin glass strip and coated with one variety of phosphor or other. Phosphor provides a wide range of color options.
Let’s not forget plain old fluorescent tubes, which are a mercury-UV “blue” UV light with a phosphor coating.
I think the Notability here is “in the wild, on the cheap” for something you’d have to actively Go Looking For to source on your own
Yeah, it is the most common way for white LEDs to be made. But using it for plain old colors like red and green is new to me.
Maybe that’s why while they come close, they don’t match them. They still always seemed blue to me and less comfortable than incandescent/halogen light bulbs.
I wish they would use a warm color as a base, not blue light, which is horrible.
The physics of how it works dictates that a higher energy, shorter wavelength like blue/uv be used. The phosphor absorbs the photons and releases lower energy/longer wavelength photons like how fluorescence works. Starting with a warmer color (I assume you mean red/yellow) as the base just wouldn’t work.
If they did this with OLED screens the colors would all fade at the same rate.
Even better, the fastest rate. Double plus good planned obsolescence.
This is exactly what they’re doing with the new QLEDs. The LEDs behind them are all blue.
It’s QD-OLED, for OLED displays. QLED displays are LCDs that use LED-pumped quantum dots for the backlight.
This is what Samsung is actually doing right now with the new generation OLED displays.. the “QD-OLED”. It is using quantum dots instead of phosporus, but the effect is the same, single color OLED light emiters converted to more colors..
That’s how LG’s WOLED process works: instead of depositing individual R G and B emitters (as with Samsung’s AMOLED process) LG deposits white OLED emitters then deposits individual filters on top of them to produce R G and B 9and sometimes Y, and sometimes W) subpixels. Newer variants intend to switch from filtered white light to monochrmatic emission and QD-based wavelength conversion, as with the old Kindle Fire HDX (jeez, was that really a decade ago?!).
Most of the cheap LEDs from China start with UV and unfortunately a lot of the UV gets out directly where it can’t be good for your eyes.
The resin the package is molded from, does not pass any harmful wavelengths, so you’re safe. Look at the packagaging on the real UVC LED’s – It’s using exotic materials to pass the short wavelength.
I hope your right as there is not a lot of resin packaging on surface mount LEDs.
Acshuuuaaally, you are both wrong.
1) Both “cheap” and “expensive” white LEDs are based on blue emitting chips. No UV emitters.
2) The LED package material still pass a substential fraction of UV-A and UV-B light which is still harmful. UV-A LEDs are no toys. UV-C LEDs are quite rare and you are unlikely to encounter them without any warning notice accompanying them.
Not all white LEDs use royal blue chips. None that I know of use UV (sub 400nm) but some do use Violet LEDs (400nm to 430nm) for high cri (97-99 cri) white LEDs.
Waveform lighting uses 420nm LEDs for their absolute series white LEDs for instance. Yujileds employs a similar wavelength violet for their vtc series.
Look like TRI-R LEDs (Toshiba / Seoul Semiconductor).
This is not an uncommon way to do this. For years white LEDs were blue doped with red (from memory) and the combo of the two colors in the right intensity made your brain see white even though the colors in the middle were missing.
It’s actually blue yellow for most white LEDs. It makes a very actinic white called moon white. Small amounts of red warm it up at the cost of efficiency.
It’s blue-yellow for “cool white”, plus red for “warm white” LEDs. It’s still made that way most of the time, and the CRI is terrible.
Yah, good luck looking for a green shirt in your closet. I discovered this after installing a nice LED light in my closet!
Yeah, probably adding a lot of uncomfortable blue light wavelength as well, that would otherwise not be present and thereby tire eyes a lot more.
Maybe that’s why they appear blue to me as my color vision is different from average, so this trick doesn’t work. Average people see blue less intensely it seems, while favoring green.
That’s the big issue with all those optimizations, they work on average, but not for everyone.
This isn’t new. Different color LEDs have been made this way for some time and is probably pretty much a requirement of long strings of lights like Xmas lights. Else all the different colors would be different brightness with the differing forward voltage of different led chemistries for specific colors.
I got an RGB flood made of blue leds and phosphor coating for each colour a few years ago. It definitely is to simplify the circuit as you don’t have to worry about voltage and current differences
Where can I get such phosphor coatings?
A lot of fractured pieces of information in a number of the comments.
There’s a wide difference in efficiency of current to lumens output for various colour/frequency/wavelength LEDs.
Some white LEDs are made with a cluster of coloured LEDs, but now most higher quality white LEDs use a driven “pump” LED that pumps/powers/excites phosphors, be they on the LED or “remote”. A few clusters are interesting in that the bulk of their ‘white’ output is from a white LED, with other colours of LED in the cluster that are driven to tune the colour output.
The typical un-powered muddy yellow colour of the phosphor mix for pumped white LEDs can be seen on the white LED “filament” bulbs, and on bulbs with an exposed white LED as phosphors on the LED or as as a remote layer in the LED’s output path (high end bulbs or mid-range like Sylvania Ultra UD).
The pump can be a blue LED (they have a lot of energy output for the energy put in compared to other colours), but the higher-end devices with a very high CRI (even 98) tend to use a UV pump for even more energy to excite the phosphors, along with filters to ensure there’s no UV output (damages displayed product or artwork, typical applications). The quality and mix of the phosphors, along with the quality of manufacture and QA binning, determines the Colour Temperature (average of the output), spectrum waveform profile and the resulting CRI.
Maybe that’s why a lot of newer LED light is very uncomfortable on the eyes, which includes car front and back lights, but also newer LCD backlight.
In direct comparison with older LEDs the difference is clear, old ones have less eye strain (and not because they got dimmer). The light quality is different, I haven’t found out what it is exactly, but I suppose it is the wavelength combined with the sharpness of the light (vs, more diffuse light in other forms of lighting).
Somewhere along the way of making LEDs more energy efficient mistakes were made. We should also focus on eye comfort, a lot of illumination today is very uncomfortable and too bright.
Filament LEDs are better, but not as good as old light bulbs yet, though pretty good. Other more common light, as mentioned, especially car lights or those flash lights on biker helmets are horrible however and blinding or very uncomfortable.
I wish regulators would focus on this way more, we can’t adapt the “hardware”, i.e., eyes to magically deal with this, like you can do with sunglasses. And even if it were possible, it should still matter at least as much as being eco-friendly.
Interesting point I hadn’t really considered before…
If a natural “red” light (or light reflected from a red object) enters the eye, it’d actually have a very wide spectrum, so the sum of all that incoming light is what we see as bright red. To simulate the same brightness with a single wavelength would mean that one-wavelength light source would have to be significantly brighter.
Somewhere in there this seems flat-out dangerous to eyes.
And, yeah, makes some amount of sense that even though they’re not “designed” to discern such a light source, eyes might experience some sort of almost “piercingly bright” sensation from such sources, even when the average intensity may be less than natural light we regularly are exposed to.
If the LED or whatever is tuned to the maximum sensitivity of our rods/cones, then almost all that significantly-more intense single-wavelength light is picked up by only one of the three color-receptors… (and, conversely, *blocked* by the other two, which creates heat, no?). Which could not only be a “harsh” sensation, but could plausibly cause other problems. E.G. causing confusion to the pupil’s dialation “circuitry.”
Yes, I agree more such studies and regulation should be done before we blind each other with oncoming headlights, blame our sleepless kids, when their rooms are lit by blue streetlights, etc.
Color mixing and coating should consider differences in color vision. Some people see yellow more vs. green, and other colors.
Having a different emphasis there is especially confusing, if you don’t have the average color vision.
But it’s more than perceptual, it’s also uncomfortable on a physical level, i.e., regarding eye strain, since the eye reacts directly and unconsciously, based on what the retina receives. Eyes are different on a sensorial level (such as red-green vision differences for example).
Obviously, there hasn’t been enough testing regarding new lighting methods, since a lot of new light, including street lamps are very uncomfortable.
I just took apart a newer flame candle with a plastic flame that has 2 leds inside .. one orange-yellow and another blue. The plastic body diffuses the leds to make you see an orange flame with a blue bottom near the wick. Anyways after tearing it down I found the 2 leds are in parallel and have the same 3.1v fwd voltage.. the yellow led has phosphor on it and is probably blue pumped while the blue Led is just native blue.. this makes the flicker circuit easy…
Did anyone ever seen the bare chips on sale? I was looking for those phosphor coated yellow and red SMD LEDs for ages, but never found one on eBay or AliExpress :(
I believe most high brightness leds are made this way, the blue are made with gallium nitride which high efficiency allows high power. You can have any color you like with the right mix of phosphors, even white (I remember reading a national geographic article, what seems like not long ago, and being completely blown away by this).
Someone mentioned, you could use this to produce oled screens that don’t change color as they burn in. This is not true as it’s the phosphors that fade and different colors fade at different rates.
Isn’t this very common. I don’t think there is anything unusual about this.
Wow….this has only been done for years now, how amazing! What is your next article going to be about, the fact that LEDs light up?!?!?!?
Wow. Just wow.
Believe it or not, it is not yet in the common-wisdom that /colored/ (as opposed to white) LEDs may (now commonly?) use this technique. They are extremely hard to find as discrete components from reputable sources, and many well-known/documented LED design techniques cannot and do not apply to devices like these.
Case in point: It has been well-established since the invention of the LED, some 60(?!) years ago, that LEDs can perform the reverse-role as photo-diodes (sensors rather than emitters), that just happen to also be best tuned to detecting wavelengths similar to their emission-wavelength. This is untrue with phosphor-coated LEDs, obviously, because their wavelength is blue/violet even though the color we see may be red or yellow. So lighting a blue LED wired as a photodiode with yellow light is nearly indetectable.
So, if you’re such a know-it-all on this front, go do something positive/constructive with your knowledge. Maybe go around looking for all the educational materials, like line-follower robot tutorials, and inform the authors that they could be confusing a lot of aspiring engineers and STEM students!