For most of us, ice isn’t something we’ve thought about in detail since our high school science classes. For most of us, we pour some tap water into the ice trays, slam it in the freezer, and forget about it. Then we lob the frozen misshapen cubes into a beer and enjoy a quite literally ice-cold beverage.
However, there’s so much more fun to be had with ice if you really get into it. If you’ve ever wondered how pretentious cocktail bars make their fancy ice spheres or transparent cubes, read on!
Heading In The Right Direction

Clear ice is the hallmark of any swanky institution; it’s far more luxurious and visually-appealing than the cloudy stuff that comes out of our domestic freezers. As it turns out, though, it only requires a basic understanding of the freezing process to make your own clear ice at home. If you know why the ice comes out frosty, cloudy, and ugly, you can counteract that to get prettier results.
Ice cubes tend to look all cloudy because of the way they freeze. The water we use to make ice has impurities and air bubbles in it. When placed into an ice cube tray, the water starts freezing from the top, bottom, and sides all at once. As it begins to freeze, the trapped air and impurities get pushed away from the frozen section and towards the center of the cube. This creates an ice cube that’s somewhat clear on the outside with a particularly cloudy center. You might have noticed that sometimes when your ice cubes start to melt, they’re clear on the outer sections and most opaque in the middle.
You may have heard of tricks to make clear ice by pulling all the air out or using distilled water, but it’s actually entirely unnecessary to use such pure water. Instead, the trick to clear ice is to pursue a technique called “directional freezing.” By getting the ice to freeze in one singular direction, the impurities in the water are all pushed to one end of the resulting cube. This creates a mostly-clear ice cube with a small section at the end that’s opaque due to trapped air and impurities. In fact, if the cube is removed from the freezer before it’s completely frozen, you can get a completely clear cube with some water left in the bottom.
Perhaps the easiest way to try this is to put a drink cooler in a large freezer, fill it with water, and leave the top open. The open top and insulated means that the water will freeze from the top down, in one direction. This tends to create a clear slab of ice with only the last quarter or so being cloudy and opaque.
Ice trays are available that use the same technique. It’s as simple as insulating the tray from the sides and bottom, ensuring that the cold air from the freezer gets the top of the tray coldest. The ice then forms from the top down, with only the bottom of the cubes showing the usual cloudiness. The trays sometimes feature an overflow reservoir at the bottom that allows the impurities to leave out the bottom of the tray to create more perfectly clear cubes. Combining these techniques with cleaner water with less air content can net even better results, but typically, just getting the directional freezing happening is enough.
Get Around It

Maybe clear ice isn’t enough for you, and you want to up your game. Ice spheres are a common way to add some pop to a mixed drink, and are fun to marvel at and jiggle around in a glass.
There are a few ways to make ice spheres, particularly clear ones. A simple technique involves using a spherical mold placed on top of a thermos. Both the thermos and mold are filled with water. The mold has a small hole in the bottom, and is placed on top of the insulated container. Much like with the cubes, the water in the uninsulated mold freezes first, with the impurities pushed out the bottom hole into the water in the thermos, which remains liquid for longer.
However, mold-based methods incur a significant aesthetic penalty in the form of a seam line. It’s possible to rub these off with some body heat, but true efficiency-heads abhor fussy post-processing steps and irregular spheres. If you want a truly perfect ice sphere, you need to get yourself an ice ball mold. These consist of two metal halves with a machined spherical cavity inside. The two halves are heated slightly, and a large hunk of ice is placed in between. The heat of the metal gradually melts away the unwanted parts of the ice as the top half slowly works its way down under gravity. The top half moves down along machined metal rods to ensure it stays true. As the two halves come together, you’re left with a perfect ice sphere inside. If you used clear ice, it’s transparent, too.
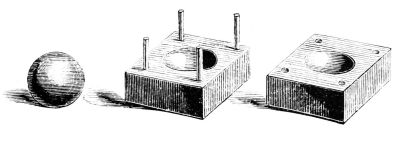
Spherical molds do have benefits of their own, too. They can make it easy to add flashy garnishes like cherries or flowers inside your ice spheres. In a particularly impressive feat, we’ve even seen a spiral-cut lime skin delicately frozen into a clear ice ball. If you’re looking for a way to justify charging $50 for a cocktail, this is it.
Kitchen Fun
Ice may seem like a humble, mundane thing. However, with a few simple tools and an understanding of the science, it’s possible to create some really beautiful effects for cocktails, desserts, or just learning about the world. Some fields of research are inaccessible for the home gamer, but playing with ice is a great way to indulge a passion for science in even the most jaded water enthusiast.
















Juat FYI, no one in Australia puts ice in their beer
Thanks, though, I don’t recall ever saying that it is a thing.
You think Lewin’s playing with us? I’ll retract my accusation!
Definitely a furphy :-)
Why? Is it because it’s so cold in Australia that you don’t need it to have a fresh beer, or because the beer is so bad that you absolutely need to finish it as fast as possible and don’t want to wait for the ice cube to melt?
We drink it cold to start with and don’t like ice ruining the flavour and getting in the way and watering down the drink at the end.
Greetings from Bavaria, beside watering down your precious beer, we have for example “Weizen” which is served with a distinctive (foam) head. Icecubes would destroy the foam crown. You won´t get Ice into your beer on any traditional Bavarian festivity (i.e. Oktoberfest). Bro-tip: Never ever ask for a Weizen with icecubes in Bavaria, you won´t leave the beer tent (or garden) in one piece
The thought alone! Is american beer so bad you need ice in it? No one in their right mind in europe is doing this. Why spoil a perfect beer? But in sodas or water with some mint leaves. Yes. And yes. Directional cooling is the trick. Just put the ice cube tray in the freezer with some styrofoam plate on top.
No, literally nobody puts ice in their beer if they value the flavor. My first reaction to that is whoever wrote this article doesn’t drink beer and just wanted to sound hip. Either that or they’re a psychopath.
I’m an American and I have met exactly one person who put ice in their beer. It’s not a thing.
Additionally, “whiskey stones” and the like are kind of a neat (ha!) way to cool your drink without diluting it, but it’s the phase change from solid to liquid that really gives ice its cooling power. Better off getting some of those slightly-less-classy-but-reusable water-filled plastic ones.
I have never seen ice in beer in the US for obvious reasons: the beer is chilled to begin with and melting ice would dilute it. And which if the thousands of American beers are so bad: number of operating craft breweries in the United States in 2022 = 9,552.
Maybe he meant to say root beer.
Best way to chill root beer is with a scoop or two of vanilla ice cream. A & W Drive-thru anyone.
Same for Canada
Simplest way to get clear ice is to drain your kettle into ice trays. Boiled water has the dissolved gases forced out. Since most kettle designs force you to boil one more mug of water than you want to use for tea or whatever, you have a boiled mug of water spare, so let it cool some then fill ice trays/molds. Being thorough with the boiling method though, I think you want to boil the water and let it simmer 5 min.
If you have an oldschool stovetop pressure cooker, you could use it “backwards” put a good fill of water in it and pull vacuum on the relief valve. Pump down, leave it a bit, pump down again, leave it a bit, pump down again. With a hand pump it’s a bit more planet friendly than wasting energy boiling it.
This doesn’t work.
One: try it.
Two. As water gets cold gas dissolved in it very well. That’s why the fish are big in Alaska.
Three. There is other stuff in tap water besides water that messes it up and is t fixed by boiling.
Done it, in hard water area, get very clear ice. If you shake the water up maybe it will redissolve air rapidly, but careful pour from an undisturbed kettle seems to work fine.
I’ve been reading a bunch about almost the opposite phenomenon: ice twigs. When an ice cube grows with a long spike sticking out of it (or in one case, I’ve seen something that looked almost like a hand, with a 3cm tall arm ending in three fingers.) Those appear to be based on a freezing plane nearly vertical to the ice cube, in the middle of it, and subsequent freezing across the top so the interior is constrained, and as it freezes solid the material with the lowest freezing point, because of impurities, gets extruded vertically in a pipe. It’s pretty rare but I see a small spike about once every couple of months, and very rarely a quite tall spike. It’s so low humidity where I am that within hours, even in the ice box, thinner spikes will sublimate away. But every once in a while you get a really interesting one.
My sister in law routinely had ice spikes on her cubes. Her daughter made investigating them as part of her science project, but to my knowledge, they couldn’t exactly figure out the cause. Has to be something in the water since I don’t see spikes an hour from them with a different water source. They moved away, so I couldn’t follow up.
Just looked them up and there’s a physical explanation. Different freezer conditions are a factor.
My guess is the tray is transferring heat away from the water faster than the air in the freezer. This causes the bottom and sides to freeze first before the top. As the top finally freezes, the ice expansion pushes water up out the unfrozen opening. Slowly building an ice chimney.
Two thoughts for a possible cause of the tray faces freezing faster than air might be a metal tray sitting on a cold thermal mass. Air vent blowing across the bottom of the tray and not the top.
Couple of decades back we had a really weird thing happening with a freezer that it turned out was going bad so was partial explanation (Sticky thermal regulation, didn’t turn on as soon as it should have or something.) The ice was climbing out one side of the trays, leaving them half empty and pooling underneath, welding them to the shelf. At first we thought of course that it was ppl being very careless with freshly filled ice trays. But with controlling that as a factor, it seemed it was similar to above explanation, with one side of the trays being “warm” and the other exposed to the cold air blower, so it would get a partial melt as temperature rose with the thermal control “sticking” (Don’t know whether it was mechanical or electronic in this instance) then I guess it crystalised toward the cold side, and then next melt cycle dripped down under the tray.
Here’s a fun overview of this:
https://www.its.caltech.edu/~atomic/snowcrystals/icespikes/icespikes.htm
Ok, now make a huge lens out of clear ice and use it for a solar cooker on the glacier.
The Edge 1997 with Anthony Hopkins. Highly recommended.
Hi. I’m Craig. Hackaday regular and inventor of the Directional Freezing Method. I originally published my work on Alcademics and provided the physics explanation as to why it works. It was an incremental advancement in the cooler method by Camper English.
My goal way a simple easy to do thing at home with no special equipment. Some years later I revisited it when my work was blatantly plagiarized by Make magazine who, after a quick letter to the editor removed the content. At that time I discovered that less than a month after I published the initial work several patents were filed using my “technology” and now, in exact opposite of what I wanted, you can buy a device to do what I shows can be done with no special equipment.
Wouldn’t nature be the inventor of directional freezing though? You thought to recreate it and share which is cool.
But why didn’t you patent it? They say. First that costs a lot of money and the entire point, again, was no special equipment. Making a clear ice ball, as I demonstrated, took a bent coat hanger and a pot of water. At the time I had just finished my PhD and was very set on intellectual honesty, attribution, as well as open source and freely available information which is why I sent my stuff to the website mentioned above.
I even wanted the method to be called the method which, obviously, didn’t stick.
And there is a fair argument that I didn’t do “much” and I readily agree it was an incremental advancement to take the slab of ice in a cooler method and reason out how to make clear ice balls. But I did do that. First. And published results and method and explanation as to why it works on the web. And now a decade later people are still doing it and there are dozens of commercial products to use the method.
I do enjoy sounding like a crank at all my swanky parties and stating I invented clear ice.
Huh. Weird formatting. I wanted the method to be called The (my last name) Method.
You could always patent a device… but you can’t patent the physics behind it.
Why such a strong attempt to hide your last name?
Belon
The Belon method.
I tried to do the hackaday weird brackets thing and it deleted it. I’m not a programmer I guess that’s… um… HTML tags for “delete this”?
Also putting your full name on a website, especially considering all the very identifiable stuff I’ve put out there seems really dumb. But here we are.
As an inventor and author, I’ve had to struggle with that. Bottom line; when your ideas get big enough, you can no longer stay anonymous online in all scenarios.
Here is my original description last name and all.
https://www.alcademics.com/2013/05/perfectly-clear-ice-balls-a-clever-trick.html
You’re aware people did invent processes to manufacture diamonds, right? It’s like, a whole thing.
Did you do any further experimentation on what ratio of ice water to “waste” water is needed? I attempted this with an ice mold and large insulated jug last night. The top of the jug froze below the ice mold before the ice mold froze. Thus, unfrozen water was trapped inside the ice mold and i ended up with a cloudy cube. It seems to me that completely submerging the ice mold would work better. Which would make it more like the commercially available molds on Amazon.
I will attempt again with square molds and a large pot tonight. But i’m curious how little wastewater you can reduce this to as more wastewater means more freezer space, less ice space, etc.
Awesome, thanks for the link. I was tempted to call bullshit on the whole story, but here we are. Are you responsible for the parrot too?
Patents are a pain in the arse and rarely “protect” your invention and certainly don’t guarantee a profit from your idea. Unless your hobby is filling out expensive paperwork I think you made the right choice. I hope your anecdote has at least impressed people at parties ;-)
The other way to “profit” from your invention would be to do engagements at swanky parties, product launches for drinks etc. That would be a sweet side-hustle. “Hi, I’m Craig, the inventor of this technique. Now’s let’s use an ice lens to set the alcohol on fire…” (or some other gimmick!)
Finally. Yeah you can’t patent physics but you can definitely patent a process (I’ve filed a couple of provisionals on processes that didn’t pan out back in my chemistry days )
This is a process. First google result
”
Processes are patentable under the U.S. Patent Act if they meet certain criteria. A process patent is a form of utility patent that covers methods of changing the functionality or characteristics of a material during a particular use.
”
I didn’t for the reasons mentioned above. And still do not want to even in the US which is a “first to conceive” country, for which I have ample documentation.
would vibrating the freezing chamber have any effect on clarity? How about low pressure?
So… this is pretty similar to “zone refining” used for purifying silicon ingots, right?
That was 100% one of the things I was thinking about. They use it for purifying gold and other precious metals too. The real insight was relating that to how ice in a lake freezes from top down (reproduced with the “cooler method”) and then using a large heat sink (pot of water) to force a thermal gradient from the top down, so the slowly descending interface forces the crud (technical term) down as water freezes, as well as just barely putting the hole in the ice ball mold underwater to both prevent the water from falling out (thank you Mr. Wizzard) as well as giving the impurities somewhere to go, all the while letting the relatively smaller ball freeze first. Also the knowledge that below about 4C, the coldest water actually loses density and “floats” to the top of the mold, again forcing top–> down freezing.
> Then we lob the frozen misshapen cubes into a beer
Excuse me, what the hell?
” lets enjoy a nice cold beer that’s been chilled and watered down with some ice cubes…..” Is this like one of those Ai chat bots pretending to be human but totally giving itself away by saying some absurdly unhuman anecdote to try and pass itself off as a normal average everyday organic homo sapien. Nice try skynet, but I’m not buying it, you can take that weak wet watery brew and spill it all up in your mother…….board..
Whatever happened to tube ice? Tube ice machines filled stainless steel tubes with water. The tubes were frozen but before the water inside would freeze solid, the remaining water was drained. Then the tubes were warmed just enough for the ice to fall out to be cut into short pieces by a spinning blade.
I don’t know if the unfrozen water just went down a drain or if it was saved and kept chilled to be used for refilling for the next cycle. That would speed up the freeze part of the cycle.
I haven’t seen this style of ice in years.