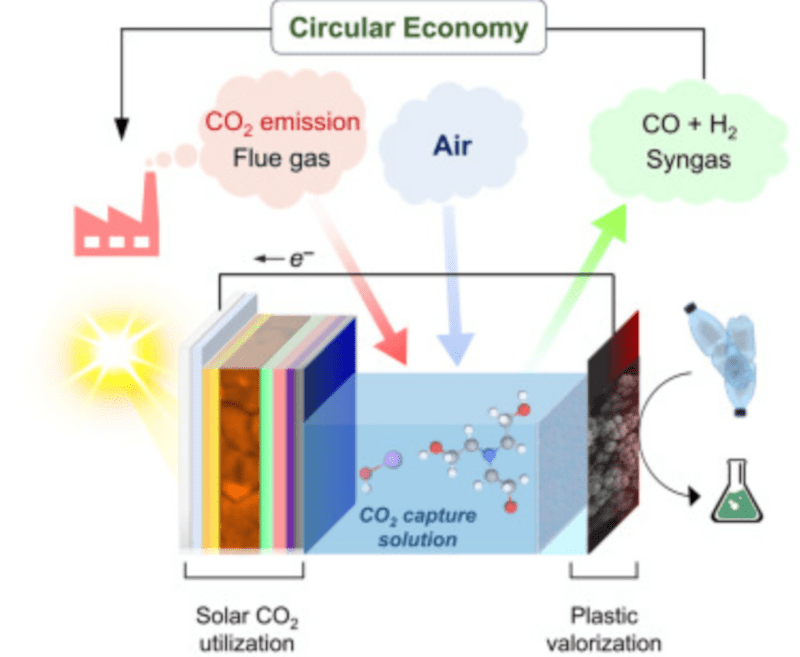
The University of Cambridge has a novel fuel cell design that can grab CO2 from the atmosphere or industrial processes and, combined with waste PET plastic, provides syngas and glycolic acid, a product used in some cosmetics. You can read about the device in a recent paper.
The strange juxtaposition of CO2 and PET is no accident. The processes work together with solar energy. There is no external voltage required, but the cell operates as a photocell to produce electricity from the solar energy. Removing both CO2 and waste plastic from the environment is a good thing.
Syngas is hydrogen and carbon monoxide and finds use in producing methanol and ammonia. It also will work as a fuel that can replace gasoline when gasoline isn’t available. It has a few other uses, like reducing iron ore to sponge iron and even converting methanol to gasoline.
The technology has a ways to go to operate at scale, and we doubt this will ever be a consumer item since you are unlikely to need syngas or glycolic acid in your home or vehicle. But it still is a promising technique to reduce both greenhouse gas and plastic waste in one swoop.
We’ve looked at other ways to grab carbon dioxide and make it useful. If you want to make your own syngas, there are other ways to do it.















We have had the ability to go from CO2, water and electricity (with no other consumable) to methane/gasoline/kerosine/ for almost a century. No PET needed.
For example not so recent HELMETH project demonstrated very nice efficiencies with ability to directly output to EU’s natural gas network and being industrialy scalable… you trade just a little bit of inefficiency compared to just H2 creation and gain access to mature transport infrastructure, buffers which can supply the whole countries for weeks and consumers from powerplants to heating to large amount of vehicles (CH4 is quite popular for use in buses in EU).
But using up plastic waste is actually a benefit of this process. And allegedly this doesn’t require electricity, so that would be another advantage. Obviously if it turns out not to be scalable or integrate-able, that doesn’t matter.
To use plastic in [Grawp]’s process, just burn it until it’s all CO2 + H2O, and you can even capture the heat to make the electricity.
I’ve been capturing CO2 from the atmosphere using solar energy and sequestering it in complex carbohydrates for many years. Lately I’ve been pyrolyzing some of it into carbon biochar, which keeps it locked away for a thousand years. I’ll let you know in August how it works on tomatoes.
+1
+1
The problem with ALL CAPS headlines is you can’t tell if they are making fuel cells from PET plastic or someone’s pet…
The source text has the capitalization “Fuel Cell Turns PET And Carbon Dioxide Into Useful Chemicals”, so it looks like no pets were harmed.
I read it the same way. Were they yelling about PETS or talking about PET plastic?
CO is extremely toxic, so that’s not something you want to play with. It’s an odorless gas that can kill you even at ridiculously small concentrations. Typically, if you have PET, you can pyrolize it to get syngas in a oxygen free combustion chamber (that can be done at home), no need for solar energy. The main issue is all the other nasty stuff in the plastic that are very very bad for your health when burnt. In the end, unless you start to build a refinery in your backyard, you better leave that stuff to people that have good lawyers.
There’s plenty of much worse chemicals than carbon monoxide (to the point of making it seem harmless by comparison) that are routinely used in chemical synthesis and even routinely transported. I’d recommend that you read up on industrial incineration, it’s very different from a domestic bonfire.
You’re saying that CO2 is not useful? Jeez, you should try living without it, or maybe you already do, like some bacteria.
What’s left out of this is the use of the chemicals needed for carbon capture in the first place. So to say this is something that you wouldn’t do in your home is a no-brainer.
Find a way to capture carbon for industrial uses without the heavy chemistry and then things like this become viable.
A recent breakthrough has done this. Search for “Faradaic electro-swing reactive adsorption for CO2 capture” for information. A local company (I know one of the employees) is making products to do exactly that: capture CO2 from atmosphere.
Since bottled CO2 is a resource at the moment, their plans are to sell units for flue capture of CO2 to carbon-heavy industry. There’s more CO2 in the furnace stacks than in the atmosphere, and the company can sell the captured gas to offset the price of the capturing equipment.
Their product will capture CO2 from the atmosphere directly, although more slowly because there’s less CO2 in the air than in flue gasses.
One can imagine putting solar farms in places where there’s lots of sunlight and little or no life (deserts or oceans) and run these cells automatically. Every couple of months or so a tanker truck drives out to collect the stored gasses to bring them for sequestration underground.
If the technology is viable in large amounts use the captured CO2 to make synthetic gasoline. Chemical energy storage is going to be around for quite a while. The physics of batteries prevent quick energize and slow discharge without significant leakage. Being able to reform water and CO2 into gasoline and other products would go a long way to make CO2 emissions a closed loop.
Something to consider when looking at using CO2 for energy transport…
CO2 is an ash. It is the end result of oxidation and energy release.
Putting the genie back in the bottle is an uphill push. Not 100% efficient – can’t be.
Putting energy into a “machine” that makes fuel from CO2 will not be as efficient as using that energy directly in the first place.