The demand for grid storage ramps up as more renewable energy sources comes online, but existing technology might not be up to the challenge. Lithium is the most popular option for battery storage right now, not just due to the physical properties of the batteries, but also because we’re manufacturing them at a massive scale already. Unfortunately they do have downsides, especially with performance in cold temperatures and a risk of fires, which has researchers looking for alternatives like aqueous batteries which mitigate these issues.
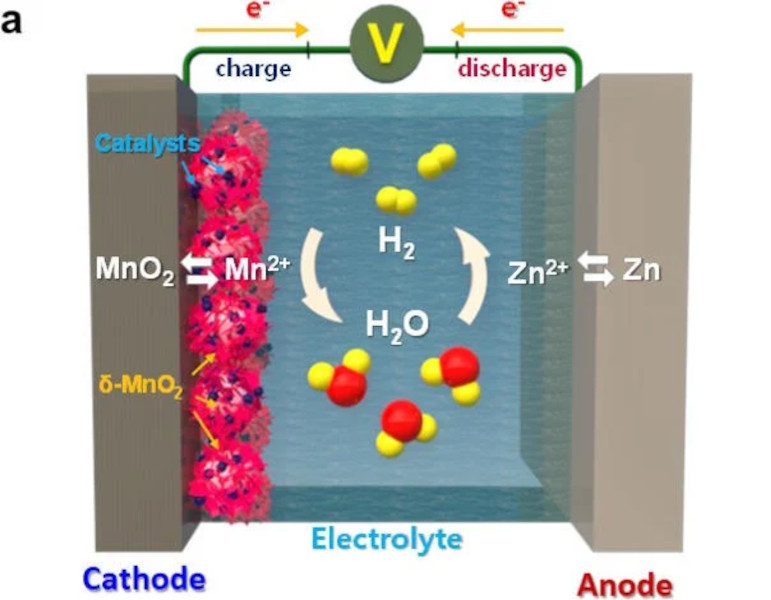
An aqueous battery uses a water-based electrolyte to move ions from one electrode to the other. Compared to lithium, which uses lithium salts for the electrolyte, this reduces energy density somewhat but improves safety since water is much less flammable. The one downside is that during overcharging or over-current situations, hydrogen gas can be produced by electrolysis of the water, which generally needs to be vented out of the battery. This doesn’t necessarily damage the battery but can cause other issues. To avoid this problem, researchers found that adding a manganese oxide to the battery and using palladium as a catalyst caused any hydrogen generated within the battery’s electrolyte to turn back into water and return to the electrolyte solution without issue.
Of course, these batteries likely won’t completely replace lithium ion batteries especially in things like EVs due to their lower energy density. It’s also not yet clear whether this technology, like others we’ve featured, will scale up enough to be used for large-scale applications either, but any solution that solves some of the problems of lithium, like the environmental cost or safety issues, while adding more storage to an increasingly renewable grid, is always welcome.















Catalytic recombination of hydrogen and oxygen has been a thing in lead-acid batteries since forever ago. Putting that tech in a zinc-carbon cell is just a waste of palladium. Enjoy your single digit charge cycles. Also, you guys should start putting the DOI in the article for this sort of thing so we can just pirate the paper without having to read tech journo slop surrounding it.
> Enjoy your single digit charge cycles.
Doesn’t matter much in seasonal scale energy storage. If a battery is charged and discharged more or less annually, you get a handful of full charge cycles in a decade. As long as the self-discharge rate and shelf-life aren’t adversely affected, it really doesn’t matter how many charge cycles the battery can handle.
DOI in the article would be nice. SciTechDaily is closer to the sensationalist end of science journalism
Why are 99% of headlines about battery developments complete BS?
They have no choice. At some point any commercial battery tech company needs external funding to get where they believe they can get (but are not yet at) to. Lies are really their only option…
It’s the disconnect between the science (we made a proof of concept in a lab and announced it because we need funding) and the 5-10 year path to actually making something in production… if it can actually be scaled at all, which a lot of stuff turns out to not be practical at scale.
But of course the internet loves to invent mad theories like they were killed by big oil or whatever rather than just their novel new chemistry didn’t work/scale in the real world.
in a word,sodium
for grid storage,weight and energy density are
not very important,abundant materials with low aquisition costs,cost per charge discharge cycle and a long service life(#cycles) is what
counts most,and bieng non flamable and non toxic will become more important as time goes
by and low environmental impact,sodium has the best bet for checking all the boxes
The obvious problem with this is that [i]palladium is very expensive[/i]. It is one of the platinum group metals. For an experiment in a lab this is fine, but it’s not going to work if we are trying to roll out batteries all over the world.
Platinum is also really expensive, but aren’t both of these materials already used (in some quantity) in other parts of the car? (Catalytic converter, spark plugs) Is the amount used in the battery vastly different than what’s already used in car parts?
Palladium (currently ~$36 per gram) is a bit more expensive than platinum (currently ~$32 per gram,) but not drastically so.
Platinum is used in catayltic converters as the catalyst. It is only a very thin plating, and it is recycled when the converters are scrapped.
Palladium would be used as a catalyst in the batteries – it shouldn’t take large amounts. I have no idea how hard it would be to reclaim the palladium from a battery.
Regardless, for use at the scale of grid storage, recycling should be a planned part of the battery lifecycle.
We’re not talking about automotive batteries that need to be light, small, and high performance.
Batteries for grid storage can be heavy and bulky and of only moderate performance. That means you can (and should) design them so that they can be disassembled with (relative) ease for recycling.
Heavy usually spells “more materials”, which spells “high price” even if the materials themselves were relatively cheap.
Eh… sometimes batteries may go that way, since you need to move ions around and if they’re both bigger and heavier, it doesn’t matter whether you buy by the pound or the gallon, you’ll still lose. But in other contexts, as an example it’s a lot cheaper to take the weight penalty and weld together a structure of cheap mild steel than to try using aircraft alloys.
This is a much more promising direction:
https://spectrum.ieee.org/flow-battery-2666672335
A company called ESS has an iron sulfur redox flow battery built into a high cube shipping container that can store 400 kWhdc. So, they’re doing just a bit better than 1MWhdc per thousand square feet. Its not to gas tank in a car levels yet but we’re getting there.
No turn key cost estimates yet though. Apparently all their projects are one offs with municipalities at the moment. I’m looking forward to the day when HOAs and apartment complexes can buy these systems to help off set energy costs. If they top off the batteries at night during off-peak hours it can go a long way to help reduce cost during on-peak hours and flatten peak load demand curves.
Their brochure: https://21814608.fs1.hubspotusercontent-na1.net/hubfs/21814608/2023-09-ESS-EnergyWarehouse-datasheet-rev4.pdf
NiMH batteries have an alkaline aqueous electrolyte, and a catalyst that recombines any built up oxygen if you trickle charge at a slow enough rate. Something like C/10 was suggested. Wonder what catalyst it is?
Elsewhere I just see mention of recombining at the metal hydride electrode; maybe they’re getting enough action from the nickel. Cheaper than palladium at least, but may explain why it wasn’t tried here if that’s what they’re doing.