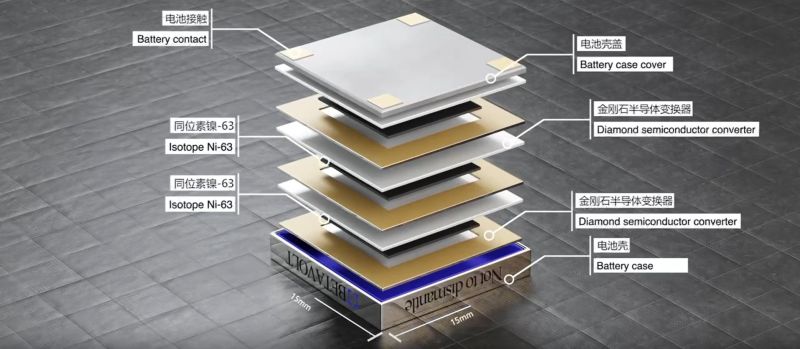
A newly introduced battery called the BV100 by Chinese Betavolt Technology promises to provide half a century of power, at 100 μW in a 15x15x5 mm package. Inside the package are multiple, 2 micron-thick layers nickel-63 isotope placed between 10 micron-thick diamond semiconductor, with each diamond layer using the principle of betavoltaics to induce an electrical current in a similar fashion to a solar panel using light. Ni-63 is a β emitter with a half-life of 100 years, that decays into copper-63 (Cu-63), one of the two stable forms of copper.
From the battery’s product page we can glean a bit more information, such as that the minimum size of the betavoltaic battery is 3x3x0.03 mm with one layer of Ni-63 and two semiconductor layers, allowing for any number of layers to be stacked to increase the power output within a given package. Also noted is that the energy conversion rate of the β energetic event is about 8.8%, which could conceivably be improved in the future.
Although this battery may seem new, it’s actually based on a number of years of research in diamond semiconductors in betavoltaics, with V. S. Bormashov and colleagues in 2018 reporting on a similar diamond semiconductor with Ni-63 isotope layer battery. They noted a battery specific energy of 3300 mWh/g. Related research by Benjian Liu and colleagues in 2018 showed an alphavoltaic battery, also using diamond semiconductor, which shows another possible avenue of development, since alpha particles are significantly more energetic.
Whether we’ll see Betavolt’s BV100 or similar products appear in commercial products is still uncertain, but they plan to have a 1 Watt version ready by 2025, which when packaged into the size of an average Li-ion battery pack could mean a mobile power source that will power more than a pacemaker, and cost less than the nuclear batteries powering the two Voyager spacecraft and all active Mars rovers today.
















I wouldn’t power a pacemaker with a radioactive battery.
Pacemakers used to be the main application for betavoltaics. The batteries last a very long time but are expensive and low power so not a very common use case.
They were replaced with lithium metal batteries as they more closely matched the life cycle of a pacemaker.
The Medtronic model 9000 pacemaker was built around an RTG (thermal) generator fueled with Pu-238 rather than a betavoltaic. The most common battery for pacemakers of that era was the mercury-zinc. There were only a small number of the model 9000 produced.
The Russian military, and probably the US, have used betavoltaics for remote sensors.
We all know: https://youtu.be/khcSeUHa5U4?t=12
They were fairly common 50 years ago, Pink Floyd named an album because of that :-) https://orau.org/health-physics-museum/collection/miscellaneous/pacemaker.html
Careful when cremating the body.
Marco’s link above states: “The hard titanium case is designed to withstand any credible event including gunshots and cremation.”
Cue Leslie Fish’s song. _Grandma Went Out With A Bang_
8.8% efficiency for a betavoltaic battery is pretty remarkable.
The proposed 1-watt battery will produce around 400 kWh over its lifetime. I wonder what it will cost…
and size, cause the 15x15x5 outputs 100uW…
I made some calculations. I’m not a physicist, so I’m not 100% percent these are correct, if someone knows better please correct me.
Ni-63 only decays into Cu-63 by beta decay, releasing 69keV of energy and an electron with ~17keV average energy (not sure where the rest of the energy goes, I guess heat). The number of disintegrations per gram per second is (ln(2)×(6×10²³/63))/(100×365×86400) = 2.1×10¹², which would be 1.44×10¹⁴ keV of total energy output per second, or about 23 mW.
So you would need around 43 gams of Ni-63 just to get a total power output of 1W, not accounting for the efficiency of the betavoltaic cell, which appears to be ~8% according to Wikipedia. That’s around half a kilo just for Ni-63, surely it will take a lot more mass to produce the complete cell. And that’s assuming the correct number is 69keV and not 17, which would multiply the mass requirements by 4. Ni-63 isn’t exactly cheap, so I don’t know about that 1W cell, it’s certainly not for consumer market.
Beta decay is a 3-body process, with the nuclei emitting a beta (high energy electron) and a neutrino/antineutrino. Only a tiny amount of energy goes into heat (recoil of the nuclei) because the nuclei is super-massive compared to electron/antineutrino (so it can’t have a lot of energy b/c it would have too much momentum).
The kinetic energy of the electron that gets emitted is continuous, from zero to some maximum energy: the two numbers you got are the *average* (~18 keV) and the *maximum* (~67 keV). So basically whatever energy the electron takes away (Ee=0-67 keV), the antineutrino takes the rest (En = 67 keV – Ee) to extremely good precision.
So basically the rest of the energy zips away in neutrinos. Heat could be useful, but sadly, it’s not heat.
Ah, I see, I didn’t think about the neutrinos. Still, I wonder wether the “less tan 10% efficient” figure of the betavoltaic cell from Wikipedia refers to the average beta particle energy or to the total nuclear decay energy. The latter would imply they can only ever be at most 25% efficient.
The efficiency is beta particle energy to electrical. It’s low because even though it’s an electron already, electrical power isn’t from higher energy electrons, it’s in the *field* – which means you need to convert that electron to *lots* of electrons. These work by the beta particle creating an ionization trail, which provides the electrons (extremely similar to solar cells). Hence the low efficiency: all the ionization also kicks the atoms involved, creating heat. Plus of course not all the betas even make it to the semiconductor.
So yeah, overall, it’s bad from a power density standpoint. The “1 W” thing is obviously marketing gimmick, as is the cell phone comments.
It is worth noting the “it’s radioactive!” fears are unfounded. These are low energy betas and can’t make it out of the cell, and can’t even damage the cell itself. So there are plenty of applications, just… not a cellphone.
I think that’s about right. I did similar calculations on the walk to work this morning and came up with about 300 grams for 1 watt (electrical), but have not sat with pencil to paper to check it.
That’s roughly 1500 Curies of activity. The vaguely similar medical isotope production process for refined Mo-99 costs around $300 per Curie. Scaling rules probably don’t really apply here due to the radically different half lives, but that implies a 1-watt Ni-63 generator will require 500,000 dollars of Ni-63. Some random google hit also tells me that Ni-63 is commercially available for $4000/gram, to that puts it at $1.2M.
Put in those terms, a ‘fuel’ cost of $3 per kWh actually isn’t that bad.
Is battery the right word here? I guess it’s an array of cells so yes in a sense, but I also tend to think of batteries as storage, while this is more akin to a generator…
It is a nuclear (radioisotope) battery, much like how a lithium-ion battery is a chemical battery. Many chemical battery types are also not rechargeable, e.g. zink-air, lithium and your typical ‘dry cell’.
It’s a primary battery, not a secondary battery.
Generator is the correct term. Battery is the maketing buzz word.
Incorrect. Generator is not the correct term.
It is a type of generator since the decay happens weather you use it’s energy or not. It’s constantly producing power and if you want to use it you can convert it to electricity.
There are other types of Radioisotope generators such as a thermoelectric one:
https://en.wikipedia.org/wiki/Radioisotope_thermoelectric_generator
The term “radioisotope thermoelectric generator” disagrees.
https://en.wikipedia.org/wiki/Radioisotope_thermoelectric_generator
You would need 10,000 of those 100uW batteries to provide 1W. That would be 50m long with the 15x15mm devices. If you increase the cross sectional area to something larger to reduce the length, you would still need 2.25m^2 volume. The diamond semiconductor would limit the cross sectional area to maybe 0.5m^2. Anyway, my point is that battery would be huge. And for only 1W. Why would they even bother?
because lots of devices require less than 1w?
And some news reports of this have claimed that it could be used for electric vehicles. Yeah, nope.
But it will be nice enough for small sensors and similar, if the cost is not excessive.
Because 1 Watts is a lot of power for something like a radio navigation beacon that protects shipping lanes and doesn’t need replacing batteries for the next couple hundred years.
Because something approximately the size of an 18650 would produce 1mW which is plenty to power a small microcontroller and some peripherals. Alternatively you could use it to charge a super cap that to drive a high power application that might sleep 99.9% of the time. You could seal that up in an IP rated enclosure and have a package you could deploy in the field that would last for decades.
This is just the diamond battery to a different tune. Just as impractical.
The perfect battery to power a nuisance beeper hidden in the ceiling of your bosses office
What if you take a beta or gamma emitter and sandwich it like a capacitor between two doped semiconductors, so that the radiation emitted causes one Plate to lose electrons, and the other plate to gain electrons, so that if you connect both plates an electronic current will flow
That’s… basically what this is…
An ESP32 in “deep sleep” mode (with the right board) consumes 130uA @3.3V or 430uW so you’d need 4 of these batteries (about $5,000 each) to keep the memory fresh for 50 years. That is if that other nuclear phenomenon Cosmic Rays didn’t flip a bunch of critical bits over those same 50 years.
nRF52840s are fairly trivial to get down to ~5uA, so it sounds like ESP32s aren’t super with sleep current. Still, I doubt this is intended for the general consumer market.
I used to work with low power radio chips (mcu + rf tranceiver) that could sleep at a couple hundred nA. So yes, those batteries would absolutely be useful for some applications.
I see this as one small step in the search for ‘practical’ long term battery technology. Who knows where this will lead in the next 300 years. I think it is a good step in the right direction though. People worry way way way to much about nuclear decay solutions….
Waiting for fattavoltaics, that would transform your body fat into electricity.
Almost lifelong supply, and some people could provide energy for several households …
“and some people could provide energy for several households …”
But, the reason they are fat, is because they are not providing energy to their own household.
B^)
“Waiting for fattavoltaics, that would transform your body fat into electricity.”
Spontaneous Human Combustion. Look it up.
Generating power with blood sugar
https://www.sciencedaily.com/releases/2023/03/230328145418.htm
H
Hmmm, some errorneous submit, anyway, back to the topic. What we need it to conquer ATP cycle.
Is nobody concerned about the activity of that thing? The label on the rendering states an activity of 50Ci, that number might not scare you but as someone who designed and built a Geiger Counter, who has worked with radioactive samples, let me tell you that this is absolutely insane and I would not want to go anywhere near this thing. This emits chernobyl-like levels of radiation. Maybe there are some applications for this in very remote infrastructure but even that is a bad idea as the Sovjet Union had to find out the hard way. There is no chance anyone in their right mind would use this in a portable device or generally in any generic application. I recommend to read about the Lia radiation incident, maybe that will help you to put the stated activity in relation.
Betavoltaics fully contain the radiation source: it’s impossible for any of the radiation to escape unless the cell’s damaged (which is a concern).
As someone who works radcon I can say this is safe because they are using nickle 63. It is a beta emitter only and decays into a stable isotope with no real gamma emissions to speak of. It also has a halflife of 100 years so it isn’t that hot.
If you do the math, ingested it will kill you 50x over. Inhaled is even worse. It takes about 67mg of the 3.3g to reach an LD50 of radiation exposure. Outside the body, though, even a plastic bottle can contain the low energy beta radiation. https://ehs.oregonstate.edu/sites/ehs.oregonstate.edu/files/pdf/rso/data_sheet_ni63.pdf
It cannot be handled like normal e-waste, that’s for sure. But overall it’s similar to strychnine. Ingesting about 100mg of that will kill a person too. Sitting in the bottle it’s pretty harmless. And the absorbed Ni levels required to be acutely dangerous are so far above background as to be readily detected without radiological equipment.
In the end the cost will limit the use to very specialized applications where stronger controls can be put into place. Accidents will still happen, but they’re not on the same scale as Co-60 medical equipment mishaps at 3000Ci of ~1Mev gamma radiation spewing everywhere.
If you take the volume of the Batt into account you can get almost 40kw a day for 50 years off 0.56 liters worth of batteries
I call bullshit on the “planned” 1 watt battery. The mass of nickel-63 to generate 1 watt of power (just using the beta particles. The neutrinos are lost energy) is 165 grams. But since after 50 years, the output would only be 70% of that, you need 236 grams to maintain the 1 watt for the rated lifetime. But, the efficiency isn’t 100%. So, assuming that they can get up to 10%, the amount of nickel-63 is either 1650 or 2360 grams (depending on if you’re talking 1 watt when new, or 1 watt at end of life). And that’s just the mass of nickel. The semiconductor matrix that actually performs the conversion and surrounds the nickel needs to be added as well. So an extremely optimistic estimate is a 1 watt battery would be approximately 2 kilograms. Just a tad heavy for a smart phone.