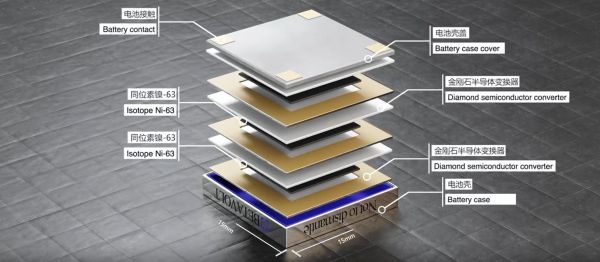
A newly introduced battery called the BV100 by Chinese Betavolt Technology promises to provide half a century of power, at 100 μW in a 15x15x5 mm package. Inside the package are multiple, 2 micron-thick layers nickel-63 isotope placed between 10 micron-thick diamond semiconductor, with each diamond layer using the principle of betavoltaics to induce an electrical current in a similar fashion to a solar panel using light. Ni-63 is a β emitter with a half-life of 100 years, that decays into copper-63 (Cu-63), one of the two stable forms of copper.
From the battery’s product page we can glean a bit more information, such as that the minimum size of the betavoltaic battery is 3x3x0.03 mm with one layer of Ni-63 and two semiconductor layers, allowing for any number of layers to be stacked to increase the power output within a given package. Also noted is that the energy conversion rate of the β energetic event is about 8.8%, which could conceivably be improved in the future.
Although this battery may seem new, it’s actually based on a number of years of research in diamond semiconductors in betavoltaics, with V. S. Bormashov and colleagues in 2018 reporting on a similar diamond semiconductor with Ni-63 isotope layer battery. They noted a battery specific energy of 3300 mWh/g. Related research by Benjian Liu and colleagues in 2018 showed an alphavoltaic battery, also using diamond semiconductor, which shows another possible avenue of development, since alpha particles are significantly more energetic.
Whether we’ll see Betavolt’s BV100 or similar products appear in commercial products is still uncertain, but they plan to have a 1 Watt version ready by 2025, which when packaged into the size of an average Li-ion battery pack could mean a mobile power source that will power more than a pacemaker, and cost less than the nuclear batteries powering the two Voyager spacecraft and all active Mars rovers today.