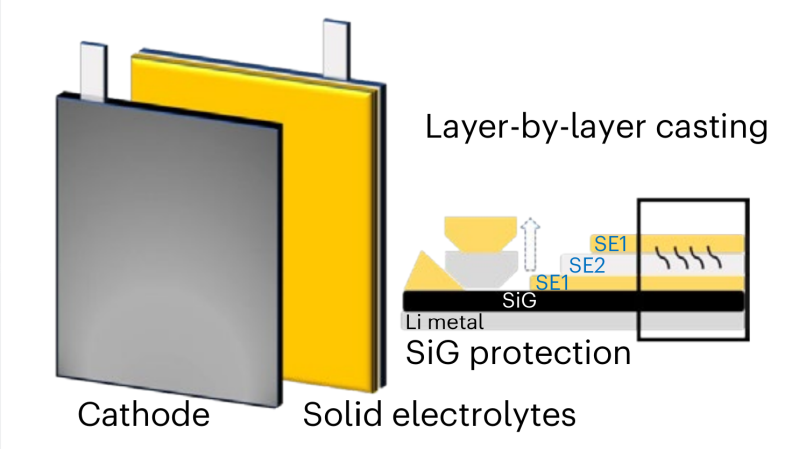
One of the biggest issues facing the solid-state lithium-based batteries we all depend upon is of the performance of the anode; the transport of lithium ions and minimization of dendrite formation are critical problems and are responsible for charge/discharge rates and cell longevity. A team of researchers at Harvard have demonstrated a method for using a so-called constriction-susceptible structure on a silicon anode material in order to promote direct metal lithium deposition, as opposed to the predominant alloying reaction. After the initial silicon-lithium alloy layer is formed, subsequent layers are pure lithium. Micrometre-scale silicon particles at the anode constrain the lithiation process (i.e. during charging) where free lithium ions are pushed by the charge current towards the anode area. Because the silicon particles are so small, there is limited surface area for alloying to occur, so direct metal plating of lithium is preferred, but crucially it happens in a very uniform manner and thus does not tend to promote the formation of damaging metal dendrites.
Current solid-state silicon-anode lithium batteries suffer from anode expansion during charging, which limits their practicality in liquid electrolyte applications. This research demonstrates that such anodic swelling can be mitigated by using silicon particles at the anode, and thus paves the way to better liquid electrolyte batteries, of the kind we use every day. In the meantime, the team demonstrated using a hybrid lithium-silicon-germanium anode structure together with multiple electrolyte layers, in a solid-state configuration. They measured improved cycling stability and capacity retention, even at very high current densities, in both coil and pouch cell configurations.
What this means in real terms — if it can be applied to the common liquid-type pouch cell — is higher charging rates, longer cell life and higher charge density. All very desirable goals.
If this research is hard to follow, we get you, it’s complicated stuff. Here’s a primer on lithium batteries to get you started, and a practical guide to acquiring and handling them once you want to get your hands dirty.















We’ve seen a lot of different ‘breakthroughs’ in batteries over the last decade – none of them are making it into production.
Yeah, all these breakthroughs in the last decade that usually take about a decade or so to make it to production, why aren’t they here dammit?
It is because manufactureres would not earn enough going From A to B as an revolution. They like to teake evolution way thro 17 generations of tiny upgrades – every time calling it a revolution.
Every upgrade to the process needs to be paid. They have to sell today’s technolgy at X% profit for so many years to fund the next upgrade in process. Meanwhile, those that just sell the old technology at X% profit take all the money home and can spend it to increase production and hone the process to be cheaper.
Which one do you think will take the market? Those who put their profits into progress, or those who put their profits into taking over the market and lowering the cost of production?
That’s why the industry moves in “revolutions”. The market is taken over by those who sell the same old stuff at ever-lower prices, until they reach the bottom price. The new technology keeps improving gradually, until it is so much better than it reaches price parity with the old standard, and then it takes over rapidly.
The step from being able to manufacture it in a lab for a single prototype for testing to mass manufacture is quite a bit step. It may not work out at all or be not reliable enough or not cost-effective.
Totally agree. Several years back, MIT developed a process to align the lithium tubes in the substrate end to end instead of scattered like they are currently. The end result was enormous efficiency, insanely high charge current, and much higher discharge current.
Oh an anecdote! Well you’ve definitely convinced me that zero innovations have made it into modern batteries despite a massive increase in capacity and decrease in material cost. /s
Well, you’re definitely wrong about that because batteries from a decade ago can’t hold a candle to batteries of today. Just because you don’t know what does and does not become integrated into battery tech doesn’t mean nothing does.
Yeah. Definitely have been improvements. Maybe they don’t all make it into every new gadget, but progress is certainly happening
Slowly. I’ve seen about 25% improvement in battery capacity at the same price point over 10 years in commodity hardware.
There are of course tradeoffs. You can get better specific capacity if you compromise on cycle life etc. which is what’s been happening with things like electric cars. Larger batteries get lower cycle numbers per mile, which affords this trade.
Although with higher specific capacity comes other trade-off, such as worse calendar life and worse fire safety, so that’s what’s been happening as well. Things like Tesla choosing to bank on NCA for the long range options instead of NMC or LFP was done over cost per kWh, not safety or longevity.
“I’ve seen about…”
Wow, what a great scientific measure meant. I’m glad we’re going with empirical evidence rather than just pulling numbers out of your oversized posterior.
You are dismissed. You are another fool who’s “information” is merely what you imagine it to be.
“the solid-state lithium-based batteries we all depend upon”
Wait, what?
Right? I’ve never seen one, nor a service using one. Pretty sure I’m not depending on them yet…
Yeah, I had to look up if that was even a thing. It refers to a solid anode of lithium. Research is ongoing, but the wiki page doesn’t mention a single company that has gone to market with it yet.
Looks like the author is mistaken or trying to throw some word salad at us.
Q: So what does this mean for all the millions of EVs on the road?
A: They’re as obsolete as a horse and a buggy.
Will have to pay for someone to take them away.
Q: So what does this mean for all the millions of EVs on the road?
A: Absoutley nothing because this is just vaporware, and the breathless use of the word “breakthrough” and “revolutionary” are just pump-and-dump marketing terms.
the crystallography makes this a useless “breakthru”
Sigh. Yet another “breakthrough” battery technology that’ll never exist outside of some university lab…
It makes me wondering about a way to increase battery life ( like AAA)
When a battery is low, I shake it in my hand and it revive it to the last electron :)
Is shaking a rechargeable battery can eliminate some dendrites ?