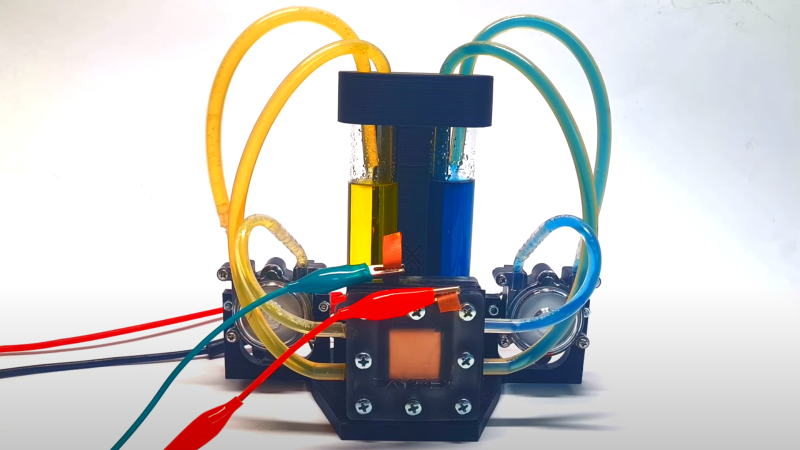
Vanadium flow batteries are an interesting project, with the materials easily obtainable by the DIY hacker. To that effect [Cayrex2] over on YouTube presents their take on a small, self-contained flow battery created with off the shelf parts and a few 3D prints. The video (embedded below) is part 5 of the series, detailing the final construction, charging and discharging processes. The first four parts of the series are part 1, part 2, part 3, and part 4.
The concept of a flow battery is this: rather than storing energy as a chemical change on the electrodes of a cell or in some localised chemical change in an electrolyte layer, flow batteries store energy due to the chemical change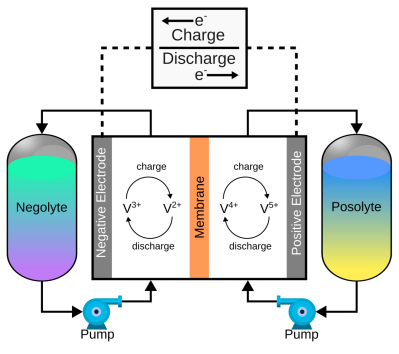 of a pair of electrolytes. These are held externally to the cell and connected with a pair of pumps. The capacity of a flow battery depends not upon the electrodes but instead the volume and concentration of the electrolyte, which means, for stationary installations, to increase storage, you need a bigger pair of tanks. There are even 4 MWh containerised flow batteries installed in various locations where the storage of renewable-derived energy needs a buffer to smooth out the power flow. The neat thing about vanadium flow batteries is centred around the versatility of vanadium itself. It can exist in four stable oxidation states so that a flow battery can utilise it for both sides of the reaction cell.
of a pair of electrolytes. These are held externally to the cell and connected with a pair of pumps. The capacity of a flow battery depends not upon the electrodes but instead the volume and concentration of the electrolyte, which means, for stationary installations, to increase storage, you need a bigger pair of tanks. There are even 4 MWh containerised flow batteries installed in various locations where the storage of renewable-derived energy needs a buffer to smooth out the power flow. The neat thing about vanadium flow batteries is centred around the versatility of vanadium itself. It can exist in four stable oxidation states so that a flow battery can utilise it for both sides of the reaction cell.
The reaction plates in the cell’s heart are printed with an ‘ABS-like’ resin for this build. They comprise a flat plate with through-holes for clamping, a central opening to house the charge collector electrodes, protection layers, and the ion exchange membrane. Pipes on the edges lead to tiny holes at either end of the flow region, on the inner edge, to enable electrolytes to flow to and from the external reservoir. Resin printing was chosen due to its strength and, most importantly, the surface smoothness, which will help to prevent leaks. The electrodes are copper sheets, with a protective layer of conductive HDPE and a second layer of graphite felt. The last layer allows electrons to be conducted to the HDPE and copper electrode whilst allowing a lateral electrolyte flow from the reservoir. A Nafion-based ion exchange membrane in the centre prevents mixing positive and negative electrolyte solutions. However, initial testing with baking paper also works for a while. Each half of the battery is filled with vanadium pentoxide and sulphuric acid.
The theoretical cell voltage is centred on 1.5 volts, but a fair current must be available even for such a small electrode area. Watching the two halves of the cell change the electrolyte state visibly during the charging process was fun. Vanadium does produce some spectacular colours in its various oxidation states! If you want to play along at home, the STL files for the 3D-printed parts can be found on the Cayrex2 Patreon site.
















Re: the headline.
ITYM “REDOX” (as in “reduction/oxidization”), not “REDUX” (as in faux-french “re do”)
I scrolled down to make a similar comment. It’s unforgivable to build at this level and not know it’s Reduction-Oxidation or “RedOx” that makes these batteries tick.
It seems like the original video title is correct (at least now), but Hackaday article just has wrong title.
Actually the author of the video suggested the title, and I forgot to correct it. I do know the difference thanks, but people make mistakes and/or forget things. Don’t have a cow man!
This is a beautiful build.
Is it possible to connect multiple cells to the same electrolyte reservoir to increase the voltage or is the electrolyte too conductive?
Yes, it’s possible, and it’s how usually is done.
One cell is low voltage, low current. You wire a set of them in series to increase voltage, and a couple sets in parallel to increase current. Or the other way around, works the same.
That’s a sci-fi device if I’ve ever seen one! Even the name sounds like Hollywood stuff. Top marks!
Agreed, I didn’t realize vanadium was an element 😂
Part of the unobtanium series.
Below Eludium on the periodic table.
The handy thing about this technology is that you can swap out the chemistry as fast as your refuel a car so for a vehicle you don’t have hours of down-time recharging as that can be supplied to you as a separate service. I’m surprised that this has not been looked into for very large vehicles that need to be kept operating for as many hours as possible, such as those massive robotic mining trucks used in Australia. Possibly also trains.
Weight and safety. You will not want to drive around with 20k liters of corrosive sulfuric acid. A truck loaded with this crashing would be a disaster. For trains you can have the battery stored somewhere and wire the trains.
Better have it stationary, so if you need more capacity you just dump in more electrolyte.
Those self driving mining trucks never leave the site (a big hole in the ground) and it is already a very dangerous place for humans. I’m not suggesting urban delivery vans or anything obviously silly.
the systems, i.e. the battery part as well as the storage, are too bulky for automotive applications (even for large stuff like the mining trucks you mentioned).
the electrolyte is something like 5M sulphuric acid. that makes it quite corrosive and a release of the toxic Vanadium species is going to happen sooner or later (especially in already tear and wear prone automotive environments).
as grid level energy storage system redox flow batteries make some sense, I’d say.
the Wikipedia article lists some pros and cons as well:
https://en.m.wikipedia.org/wiki/Vanadium_redox_battery
The HQ of Telstra in Australia has had a flow battery as a backup since the early 1990’s (not sure what is there now though), I lived around the corner in Melbourne and recall seeing it. Was the size of a shipping container. There was a bit of oooh-aaah in the traditional media about it but it was pre-internet so just local TV and or papers, remember when they would put black marks on sheets of dead tree fiber? Ah those were the days…
The problem with battery technology for big power demands is that as the requirements increase so to does the mass of the battery. And full or empty that mass needs to be dragged about, which in terms of efficiency for moving a heavy load between fixed points, carrying extra mass is sub-optimal. Probably a rethink would be better like overhead tram lines for electric mining trucks and a much much smaller battery for moves between overhead tram lines. That way the advantages of battery power are not lost dragging expensive dead weight about. You could probably even design it that trucks traveling downhill provide partial regenerative power to trucks going uphill.
A truck traveling downhill to generate energy is exactly what this elevtric mining truck does https://insideevs.com/news/361095/edumper-largest-ev-world/
Because it travels downhill fully loaded and goes back up empty all day, it actually generates energy which gets fed to the grid during the nighttime.
Not that it’s the right application, but freight locomotives are intentionally made heavy for traction, etc. It might be more of an issue for the extra volume than the mass.
There is a English sports car company already doing this.
Interesting. Does it produce more energy than the pumps need? A dual chamber single motor pump might be useful as both chambers will use up electrolyte at same rate.
One this small probably won’t, but looking at commercial versions, you wouldn’t need to scale it up much before you can self power.
The project is very well built, and just by watching it operate I guess it would be possible to detect the state of charge with colorimetric methods. Maybe just an appropriately colored LED and photodiode.
However, please remember before jumping into a new chemistry project to always check the material safety data sheet of the involved reagents (and products). And sure enough, vanadium pentoxide is indeed quite toxic. I would not recommend to replicate this as a first chemistry project.
Nobody here seems to be disturbed by the need of outside power to charge or operate this accumulator (it is not a battery since there is only one cell). I don’t know if this can run its own pumps, but anyway this means that you get less power than you stored.
If the various vanadium oxides have different density (and with a large enough margin), you can ditch the pumps and locate the ion exchange filter at the propper height in the oxide tanks so only the best reacting oxide will wet it. This means that the tanks will not be at the same heigth relative one to the other (like the letter H, but with the vertical bars moved up or down and the ion exchange in the horizontal bar position).
You would expect someone reading this to be disturbed by it not being a perpetual motion machine? As it doesn’t look to consume anything at a glance.
Unless I’m missing something given conservation of mass I’d assume quite the opposite!
You didn’t understand my point.
1. you need extra power to run the pumps when you need to use the accumulator.
2. the accumulator (or a battery of) may not have enough power to run the pumps in order to use it independently, in which case you dont need such a solution because you waste more energy than you get out of it.
3. I proposed a way to run it without pumps.
4. I did not complain that it ain’t a perpeetum mobile. I know they cannot exist.
You always get less than you stored, there are losses even in normal batteries. Larger losses are expected in many grid storage solutions, where other statistics matter more.
If density differences could passively pump it fast enough to work, then an active pump of a similar speed would not be expected to require very much power. If a reasonably efficient pump was discovered to require too much power, that would imply passive pumping would not be able to match the flowrate anyway. That would prompt a redesign.
Actually, it’s far more efficient to move the fluids via Lorentz force. By subjecting a properly shaped and plumbed cell to a magnetic field, the fluids can be moved between storage and cell without a mechanical pump.
Research how to conduct the flow without pumps by changing the cell shape and placing the cell within a magnetic field. As the electrochemical exchanges occur within the cell subject to a magnetic field, the fluids will rotate and if subject to a routing method about the perimeter of the rotation, then a non mechanical pump can be created. Intakes introduced center axis of the routing fluids while exhaust occures about perimeter fins designed to capture the outer radial fluid motion, sending it back to the storage tank.
So in effect, you can use the swirling motion of a fluid undergoing electrochemical exchange within a cell subject to a magnetic field as your pump system. This increases the efficiency of the system significantly while simplifying the design and reducing the build costs.
When you see this post : Oh yes cool project to build, especially with solar panels !
When you see 20 Wh/kg of specific energy : Arf… Maybe not…
Could a similar setup be used for an iron redox flow battery? That would be nontoxic and thus much more useful as a demonstrator, especially for kids
Yes! The cell setupe for the all iron flow battery is the same. The vanadium redox flow battery was made because many people asking for. But yes,…. all iron flow battery based on iron chloride can be made that way…. FeCl2/FeCl3
Here’s a wiki article related to your question and the answer is, yes.
https://en.m.wikipedia.org/wiki/Iron_redox_flow_battery
There’s a company out of Oregon called ESS that has an iron salt redox flow battery.
Their documents claim they can squeeze 400kWh worth of storage into a standard 40x8x9.5′ high cube shipping container. So they’re getting better than a MWh worth of power per 1000 square feet. No too shabby. Especially considering they don’t turn into an exothermic self oxidizing chemical fire if you poke a hole in them.
The average home in the U.S. can run for about 5 weeks on 1 MWh of electricity. I’ve also read some reports that on-peak power consumption in places like central Texas works out to about 100 homes per megawatt hour of energy. Given the wide variation of what a “home” is and how efficient said home might be I have to take those numbers with a grain of (iron) salt.
I’ll see myself out.
I’d say that the home consumption average could be right given that the average home isn’t purely electric and isn’t in an extreme climate. 2.5 MWh/month in the top two months of the year (and much less in minimum months) is an example of what you might get when all your appliances are electric, all your heat is resistive, and you need lots of air conditioning in summer yet also portable heaters in winter to protect pipes and your wellhouse. And you cook at home.