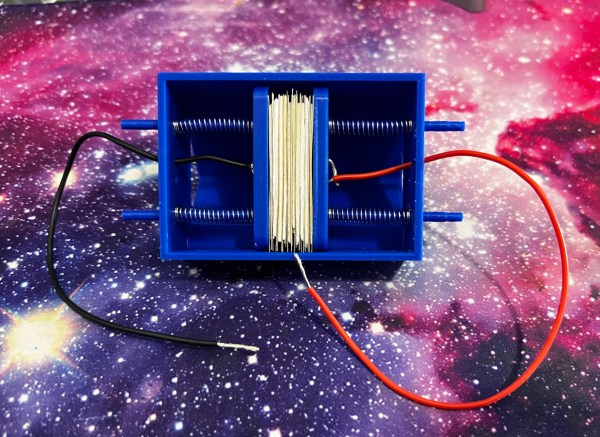
Most research on electroplating tries to find ways to make it plate parts more uniformly. [Ajc150] took the opposite direction, though, with his selective electroplating project, which uses an electrode mounted on a CNC motion system to electrochemically print images onto a metal sheet (GitHub repository).
Normally, selective electroplating would use a mask, but masks don’t allow gradients to be deposited. However, electroplating tends to occur most heavily at the point closest to the anode, and the effect gets stronger the closer the anode is. To take advantage of this effect, [ajc150] replaced the router of an inexpensive 3018 CNC machine with a nickel anode, mounted an electrolyte bath in the workspace, and laid a flat steel cathode in it. When the anode moves close to a certain point on the steel cathode, most of the plating takes place there.
To actually print an image with this setup, [ajc150] wrote a Python program to convert an image into set of G-code instructions for the CNC. The darker a pixel of the image was, the longer the electrode would spend over the corresponding part of the metal sheet. Since darkness wasn’t linearly proportional to plating time, the program used a gamma correction function to adjust times, though this did require [ajc150] to recalibrate the setup after each change. The system works well enough to print recognizable images, but still has room for improvement. In particular, [ajc150] would like to extend this to a faster multi-nozzle system, and have the algorithm take into account spillover between the pixel being plated and its neighbors.
This general technique is reminiscent of a metal 3D printing method we’ve seen before. We more frequently see this process run in reverse to cut metal.


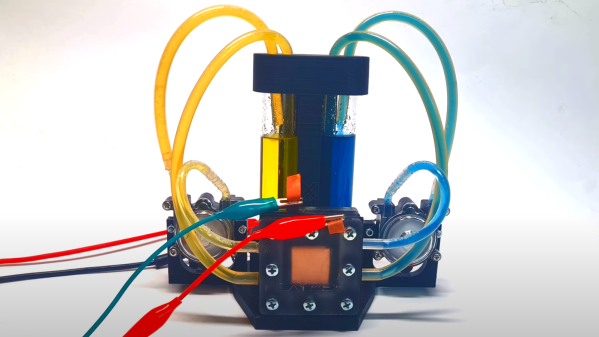
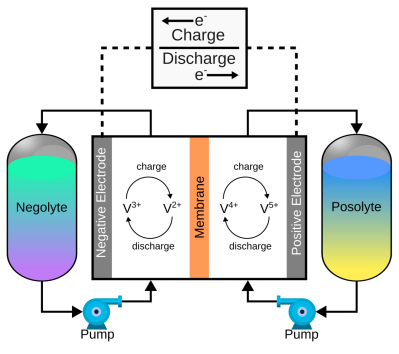 of a pair of electrolytes. These are held externally to the cell and connected with a pair of pumps. The capacity of a flow battery depends not upon the electrodes but instead the volume and concentration of the electrolyte, which means, for stationary installations, to increase storage, you need a bigger pair of tanks. There are even 4 MWh containerised flow batteries installed in various locations where the storage of renewable-derived energy needs a buffer to smooth out the power flow. The neat thing about vanadium flow batteries is centred around the versatility of vanadium itself. It can exist in four stable oxidation states so that a flow battery can utilise it for both sides of the reaction cell.
of a pair of electrolytes. These are held externally to the cell and connected with a pair of pumps. The capacity of a flow battery depends not upon the electrodes but instead the volume and concentration of the electrolyte, which means, for stationary installations, to increase storage, you need a bigger pair of tanks. There are even 4 MWh containerised flow batteries installed in various locations where the storage of renewable-derived energy needs a buffer to smooth out the power flow. The neat thing about vanadium flow batteries is centred around the versatility of vanadium itself. It can exist in four stable oxidation states so that a flow battery can utilise it for both sides of the reaction cell.