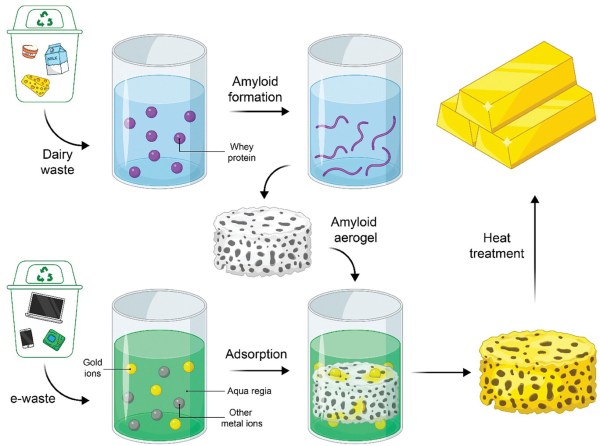
A big part of the recycling of electronic equipment is the recovery of metals such as gold. Usually the printed circuit boards and other components are shredded, sorted, and then separated. But efficiently filtering out specific metals remains tricky and adds to the cost of recycling. A possible way to optimize the recovery of precious metals like gold could be through the use of aerogels composed out of protein amyloids to which one type of metal would preferentially adsorb. According to a recent research article in Advanced Materials by [Mohammad Peydayesh] and colleagues, such aerogels could be created from protein waste from the food industry.
The adsorption mechanism of the protein amyloids is a feature of these proteins which form chelants, which are structures that can effectively bond to metal ions. These are usually organic compounds, and are used in certain medical treatments where heavy metal poisoning is involved (chelation therapy). By having these protein amyloids in an aerogel structure, the surface area for adsorption is maximized, which in the research article is said to have an efficiency of 93.3% for gold recovery, while leaving the other metals in the aqua regia solution (nitric and hydrochloric acid) mostly untouched.
Of note here is that although the food waste protein angle is taken, the experiment used whey protein. This is also one of the most popular food supplements in the world, to the point that microbial production of whey is a thing now. Although this doesn’t invalidate the aerogel chelation approach to e-waste recycling, it’s a curious omission in the article that does not appear to be addressed.