At this point, atomic clocks are old news. They’ve been quietly keeping our world on schedule for decades now, and have been through several iterations with each generation gaining more accuracy. They generally all work under the same physical principle though — a radio signal stimulates a gas at a specific frequency, and the response of the gas is used to tune the frequency. This yields high accuracy and high precision — the spacing between each “tick” of an atomic clock doesn’t vary by much, and the ticks cumulatively track the time with very little drift.
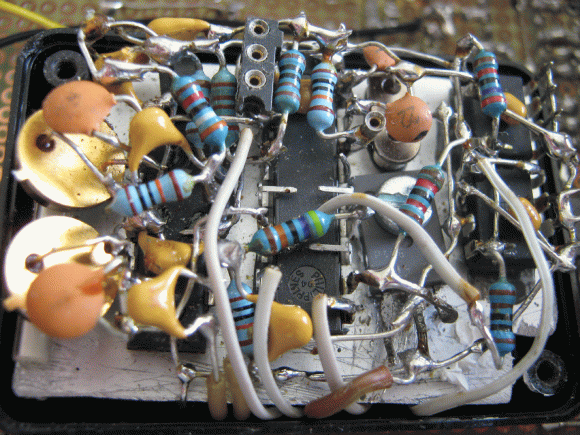
All of this had [alnwlsn] thinking about whether he could make an “atomic” clock that measures actual radioactive decay, rather than relying on the hyperfine transition states of atoms. Frustratingly, most of the radioactive materials that are readily available have pretty long half-lives — on the order of decades or centuries. Trying to quantify small changes in the energy output of such a sample over the course of seconds or minutes would be impossible, so he decided to focus on the byproduct of decay — the particles being emitted.
He used a microcontroller to count clicks from a Geiger-Müller tube, and used the count to calculate elapsed time by multiplying by a calibration factor (the expected number of clicks per second). While this is wildly inaccurate in the short term (he’s actually used the same system to generate random numbers), over time it smooths out and can provide a meaningful reading. After one year of continuous operation, the counter was only off by about 26 minutes, or 4.4 seconds per day. That’s better than most mechanical wristwatches (though a traditional Rubidium atomic clock would be less than six milliseconds off, and NIST’s Strontium clock would be within 6.67×10-11 seconds).
The end result is a probabilistic radiometric timepiece that has style (he even built a clock face with hands, rather than just displaying the time on an LCD). Better yet, it’s got a status page where you can check on on how it’s running. We’ve seen quite a few atomic clocks over the years, but this one is unique and a great entry into the 2025 One Hertz Challenge.






 Fortunately, this is a problem that’s going away on its own, so the headline is really only appropriate as a great reference to a
Fortunately, this is a problem that’s going away on its own, so the headline is really only appropriate as a great reference to a