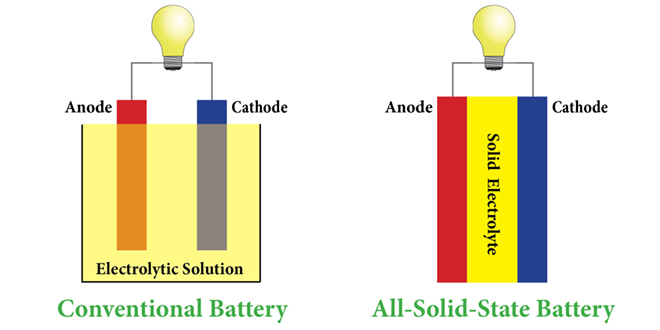
Researchers from MIT and the Samsung Advanced Institute of Technology have been developing a new material that could potentially revolutionize the battery industry. A solid electrolyte that won’t wear out, lasting exponentially longer than current battery chemistry.
It also has the possibility to increase battery life, storage, and the safety of batteries — as liquid electrolytes are the main reason batteries catch on fire.
Sound too good to be true? The idea for solid-state batteries has been around for awhile, but it sounds like MIT and Samsung may have figured it out. The current materials used for solid electrolytes have difficulty conducting ions fast enough in order to be useful — but according to the researchers, they’ve discovered formula for the secret sauce. They’ve published their findings on Nature.com, which is sadly behind a pay wall.
Another great benefit of solid-state batteries is they would be able to operate at freezing temperatures without a problem. What do you think? Is Samsung blowing smoke, or will they actually release a battery you never have to replace?















One problem solid electrolytes have had is enough current density to allow for large loads, such as in vehicles. That said, there are many applications where a slower rate can be used.
Isn’t that the same as “The current materials used for solid electrolytes have difficulty conducting ions fast enough in order to be useful”.
Yes, it shows up as internal resistance as the current grows, so energey is wasted as heat in high current applications
A battery you’ll never have to replace ?
No.
How will the company make more money?
They need recurring revenue.
Products are designed to last as long as the warranty. Anything else is a bonus.
It’s all about profit.
How many do you think they would sell if they’re rechargeable and enable electric cars? Currently Samsung gets very little profit from the extremely large oil and transportation industries. I’m sure they would be happy to get a fraction of that profit, especially considering they don’t currently sell batteries either.
Just as a point of fact, Samsung does sell lots of Li-Ion batteries. Of the Asus, HP and Dells I’ve seen, they’ve had Samsung cells inside the case.
Samsung 18650 cells power the Tesla Roadster. With 70% of the Android phone market and lots of the camera and camcorder and laptop markets, Samsung can’t afford not to make batteries.
Yes, but the Tesla Roadster is discontinued and I believe Tesla and Panasonic are married now on 18650 cells.
This is not correct. Elon Musk mines and manufacturers his own batteries for the new Tesla’s Model S, not including he released all the information about that technology to be used for free by other manufacturers.
Ah, didn’t realize they were actual Samsung kit – assumed they were rebranded OEM stuff. Thanks for the heads up!
Who said anything about rechargeable ?
it would be pretty dumb not to make them so.
About mas dumb as it would be to make them “last a lifetime”
I think you missed the point.
No company in their right mind is going to make something that lasts forever.
Other than the nuclear waste industry perhaps.
Right because my 1920s sears wrench set doesnt exist. And even my newest sears cresecnt wrench doesnt have a lifetime gaurantee and sears doesnt replace them for free no questions asked because they are designed to fail. This level of cynicism is ridiculously stupid.
+1. All it takes is a larger profit margin to extend the development cycle, to produce something new for returning customers, not including calculation of market saturation.
The way I’d do it is simple: Make them available as solder-down only. The batteries can last forever, the products they’re in don’t have to. I’d be able to simplify the design by not having to worry about planned obsolescence, which would hopefully reduce costs a bit, and I’d be able to sell as many as are currently being sold for internal applications.
Because no one will figure out a way to make a socket for soldered down parts… ;)
Doesn’t matter to the companies that don’t care about having replaceable batteries. Think of all the never-replaced batteries that OEMs have to either purchase sockets for, or already solder in. They gain diddly for making it replaceable, those are the companies to market to. Any extras sold due to whatever other reasons are just that, extras.
The downside is this relies on the batteries being cheaper to produce.
Just incorporate obsolescence in the charge controller firmware, if that keeps them up at night.
Well, how e.g. hammer companies make money? How often do you break a hammer? And does a new one cost you over the top?
All the time. I’ve owned more hammers than power drills. The claw on the back of a hammer wears out or cracks off, but usually the head rips straight off after a heavy swing.
I can’t say I’ve ever worn out or broke a hammer, and I use mine somewhat frequently. the only hammers I have worn out are soft material types, like dead blow and plastic faced ones.
So Salec has a point. Companies that make tools that for most rarely wear out still make more hammers every day and sell them. They also, like Samsung have other products in their line up that require replacement more often.
Screwdrivers on the other hand… I’ve gone through a lot of those….
That’s why you shouldn’t buy hammers, but only the heads and fit a wooden handle to it. The head is practically forever if you don’t misuse or lose it. If you break the shaft, just whittle a new one.
The cheap $10 hammers you buy are built with a tube that shears off at the neck after so many hits because of metal fatigue. It’s basically built-in obsolescence. If you buy one for fixing a few things around the house, it will last you a lifetime, but in any sort of professional work, you’ll break three and then buy the more expensive model. Then you break two of those…
i haven’t seen a hammer like that sold in years. they all have fibreglass handles now.
Estwing makes some of the best hammers in the world. If you have the right one for the jobs you are doing and don’t use it for things it wasn’t made for, it should outlive you by a few generations. I like the all metal ones that are forged from a single piece. Fiberglass will start to wear on the neck, just below the face if you miss a lot on your swings.
A lot of people buy one hammer and use it for things it was never intended to do, which is OE, not equipment failure.
The head of the hammer wears too.
everyone can make a hammer but just few factories can make working li-ion cell that has decent capacity
goods that can be make by anyone can’t be overpriced, but if you need new battery for your mobile and have choice of 2-3 suppliers they can set price anywhere they want
And they should be called out out for the criminal price gougers they are and put in prison. ;)
so, you’re saying that an artisan who provides unique goods should be payed the same amount as a day laborer? swordsmiths made more that farriers because swords are harder to make. if anyone could make a sword then they wouldn’t be worth more. it’s basic economics.
Outside of consumer electronics, there are application areas that you want extra long life if when you can’t get to the battery easily or that replacing the batteries in the field can be very costly. e.g. implantable electronics, space, emergency, some industrial applications, military etc
A battery you’ll never have to replace?
No, but you’ll want to replace what it is powering.
How will the company make more money?
Sell the battery to laptop, electric car, and cellphone companies. How many people replace their phones every one to two years?
everyone with a two year contract, and every six months for iphone users. also samsung, now that i think about it.
+1
Monopoly forces are responsible for this anti-competitive tendencies in modern advanced capitalism.
Yeah! That is what it says on my on my hammer. Go Bernie!
*Pay for the blades bro, pay for the blades.*
The shaving handle aka razor cost next to nothing. It even has a gently pulsing motor to making shaving smoother. But DAMN homey, replacement blades cost a $h1t ton.
That is the model. Bic and Gillette forever at war.
Truth be told, Yes, they can make a vanadium/iridum razor that you would never have EVER buy again.
What about a Carbon Fiber and Gorilla Glue or Liquid Nails Epoxy – Car Tire that would never need replacing? Yup, Company would rock profits for 10 solid years before, no one would even need a Tire for the life of the car/vehicle again. Them what? Goodyear gone, Firestone gone, and our pimp mega-tech tire company gone as well.
I agree… FOR CIVILIAN applications this has no value. For Mil-Spec??? hell Darpa would love you. A solid state battery that never expired? Damn Bro.
A battery that has been in a vault for 50 years that is compatible with Generation Y Exosuit (Jacket/Edge of Tomorrow)? You’d get a honorary Ph.d and a permanent seat on a Emergency Solution Council. Hell if you were honest, honorable and stalwart in your work ethic and personal life; a 5 star general would GIVE you his daughter.
So, hate to say it, yeah we are profit driven. But the hell with it, if you got the chops and the drive. Unless you got super luck or super rich, (sorry, we don’t since we are posting in this forum/thread/dreamland/lotus-eaters dream land).
Do It and get a GREAT lawyer to protect your ball sack.
Lol made up mythical tires that companies hide from us for profit. They probably have the water car patents too lol.
That’s also why tin-foil companies make their product so it wears out and you have to keep buying new hats.
Though you can buy an EMC shielding room and it will last for pretty much forever. Could be useful for dave.
Permanent batteries in cellphones and laptops would be a competitive advantage for those offering them. They’d allow the “closed case” without so much objection, and you’re going to replace those devices anyway. They would also win in EVs — the assurance that EV batteries will never die would sell more EVs (assuming a reasonable specific energy) than anyone’s making with replacement EV batteries. There are plenty of things that fail in all of these, other than the battery.
And sure, if Samsung was the only company making Li-ion batteries, they could think about either holding this back or charging crazy money for it. But Samsung SDI only have 19% of the market. If they had an immortal battery and Panasonic and the others didn’t, that additional business would put them on top, completely dwarfing that tiny, tiny replacement battery business.
It might make a few appearances, then go the way of the Edison battery.
Do telephone companies still use Edison batteries for backup?
NYC subway system still uses a bunch of them. So do many other industries……. Its gotten to the point where they are being manufactured new instead of a bunch of people refurbishing and selling them. There is a decent movement for solar installs using new Edison batteries. They may weigh allot however durability is the key. If your investing 70k in an off grid home, you don’t want lithum or led acid batteries. These solid state batteries would be a direct replacement to those edison cells for people and industry that needs durability.
Edison batteries are notoriously slow to charge and have poor efficiency because of the overpotential required to charge the cell. They typically lose 40% of the input power, so you need to install 2/3 more solar panels etc. to run the house and that gets mighty expensive.
I assume there’s been little or no research and development of Nickel-Iron batteries since Exide Battery Corp. bought and shutdown the Edison Storage Battery Company in 1972. Wikipedia claims that they were quite profitable for Edison, but OTOH why would a company like Exide that makes expendable Lead-Acid batteries want to make a battery that never needs replacing?
It’s too bad NiFe batteries haven’t had the 40+ years of R&D that NiCd, NiMH and Li-ion batteries have had in the mean time. Who knows how much room there is for improvement.
James sorry but when they where referring to indefinite storage they meant no leakage current. Not that you can recharge these an infinite number of times. Such a poorly written article anyone could make that mistake.
The problem is not only the electrolyte but also the Anode and Cathode. They are blowing smoke anyway, these exotic ceramic solid electrolytes aren’t cost effective, neither are all the amazing whiz bang battery/supercapacitors you hear about being made with thin film deposition. They don’t scale to production, it works with semiconductors as they are tiny with a very small surface area. A new battery must be designed from the ground up with production primary and performance secondary.
By poorly written article I mean the computer world one, as with all these tech ravings from people who have no idea how things work it was a cringe fest.
“James sorry but when they where referring to indefinite storage they meant no leakage current. ”
No, they also mean high recharge cycles – no, not infinite, but thousands of charge/discharge cycles might as well be infinite. It’s even right in the first paragraph. “Indeed, solid-state batteries that retain almost full storage capacity over thousands of cycles have been demonstrated.” And the references there show batteries built with as high as 50,000 cycle charge retention. Even with 1 full charge/discharge cycle a day, that’s more than a lifetime, which was the entire point.
“They are blowing smoke anyway, ”
Well, *this* paper in particular isn’t blowing anything, since they aren’t claiming to have built anything at all. They’re just showing what’s needed in the lattice structure to get the highest performance out of solid-state lithium ion batteries. Someone still needs to figure out a compound that actually has that structure. Then someone needs to actually find a way to make it efficiently. So yeah, obviously, you’re way far off. But the entire point of the paper was that previously, the compounds had basically been a random walk, whereas this paper identifies exactly what *direction* you need to go in for material development.
Yes they are blowing smoke, build the thing and test it. It will never perform to theoretical limits.
It will however have very low leakage.
It will never compete with super-capacitors in terms of cycle charge retention.
It will also never compete in energy density with lithium sulfur silicon batteries. Stanford built one in 2011 you bet your hiny they are working on a better one as am I. It doesn’t need 50,000 charge cycles to be disruptive only around 2,000. Why use exotic electrolyte when the anode and cathode break down in short order?
Also simply close the recycling loop and who cares how many charge cycles it has if only sufficient, and having 5x the energy density.
Obviously you are way far off if you don’t get that. All chemistry boils down to experimental testing not computer simulation, original article was buzz-feed bull hock.
“Yes they are blowing smoke, build the thing and test it.”
No, they’re not. There’s nothing to test. They aren’t even talking about any specific material at all.
“All chemistry boils down to experimental testing”
Experimental testing verifies models, but fundamentally, all *discovery* boils down to model-building.
If you don’t have a model for how things work, you might as well grab random things off the street and shove them together. Why not try banana peels and evaporated Mountain Dew? It might work!
Oh wait – you wouldn’t do that because you know that wouldn’t make any sense. Because you have a basic idea as to how chemistry works. You know that batteries need to be able to store/liberate ions at the cathode and anode. You know that the electrolyte has to allow those ions to move. That’s a model for how a battery works. It’s a ridiculously simple model, though. Chemists obviously have better models in their head – in their instincts, built from experience.
This is just another model, that’s all. It’s not useless, it’s not groundbreaking. It’s just a model, to aid in the discovery of new battery chemistries.
“Also simply close the recycling loop and who cares how many charge cycles it has if only sufficient, and having 5x the energy density.”
There are a ton of reasons to want high cycle count and high safety factor at the expensive of energy density. Just because most consumer applications wouldn’t want it doesn’t mean its useless.
Here I am re-organizing my sources, I think we are on the same page just misunderstanding each other.
https://hackaday.io/project/4726-artificial-muscles-and-li-s-si-ultracapacitors/log/24070-refining-the-morphology
I will continue to update throughout the day when I get time.
It’s not in Nature! It’s Nature Materials. One of their money making spin offs. The main point of this battery is very low self-discharge. Expect all other characteristics to be worse than current technology.
Thanks for clarification.
Usually lab batteries start as better in a specification and worse on other statistics, so there is hope for improvements.
On the subject of batteries i read this story about new hydrogen batteries that charge an iphone for a week. Sounds fake but I thought I would share anyway. http://www.telegraph.co.uk/finance/newsbysector/mediatechnologyandtelecoms/11818151/Revealed-the-first-hydrogen-powered-battery-that-will-charge-your-Apple-iPhone-for-a-week.html
I have seen cells that use ethanol used with air and a catalyst to last a few weeks on laptops and cellphones, and you add more alcohol to recharge. This was 6-7 years ago, and they never made it mainstream, but I understand they use them on ocean bouys and other isolated places.
Cool, I think they would need to be idiot proof before going mainstream and that’s prob why they never did, Probably have a few extra hazards using hydrogen or ethanol.
These cells are very sensitive to catalyst poisons, the ethanol has be free of certain contaminants, which would be expensive to do on a large scale…
More like the contaminants added to denatured alcohol probably won’t work here and I doubt anyone would want their fuel taxed the same as hard liquor.
Hydrogen cells aren’t new and have terriffic performance in some respects. They are expensive, fragile and explosive though.
Hey thanks, I just couldn’t trust the media as they claim new products like this all the time that turn out fake.
On the subject of “products […] that turn out fake”: »We have now managed to make a fuel cell so thin we can fit it to the existing chassis without alterations and retaining the rechargeable battery.« (quote in the article you referenced above) seems to be a rather tall tale. Apple lists the iPhone 6 as 6.9mm, iPhone 6 plus as 7.1mm. Obviously they care greatly about differences in the 0.1mm range. If there really were any excess space in the case where a fuel cell would fit, you’d better bet your ass that the Apple engineers would’ve gotten rid of that in order to make the case 6.8mm thin.
There is no money in making things that don’t wear out. The demand would fall off and eventually make it not profitable to stay in that business.
Ofcourse there is. Open any piece of electronics. Apart from electrolytic caps and batteries, just about nothing else ever wears out and those companies are doing just fine.
People buy new products and new products will use new batteries. Better batteries will mean new product categories and they will make renewable energy and electric cars more competitive.
Every sane company will want to own that technology.
At my workplace I could access the paper… and now I regret reading it!
The authors have done CALCULATIONS (DFT and ab-initio MD as far as I can tell) on what material properties could be beneficial to the conduction of Li+ ions inside the solid electrolyte. What they found is, that a body-centred cubic-like anion framework seems to improve the Li+ conduction.
In the paper there is no claim of everlasting batteries… and as some comments already state, there is a LOT of other factors influencing the lifetime of a complete battery system.
As always, if someone gives you a simple answer to an utterly complex problem of any kind, it’s nonesense! From my empirical studies (aka life) this is true for science, politics and the universe itself…
42
For people unable to get a University IP address to access that content:
> In summary, our study highlights the critical influences of the
> anion-host matrix on the ionic conductivity of solid-state Li-ion
> conductors.
In essence, the article is not about new fancy solid-state batteries (which is not surprising at all, it’s a concept as old as the battery), but about new lattice materials.
You shouldn’t write articles about articles which you can’t read.
https://news.mit.edu/2015/solid-state-rechargeable-batteries-safer-longer-lasting-0817
It’s a solid-state electrolyte. Close enough.
It’s not about new lattice materials – it’s about *how to find* new materials. The important quote from the paper is this.
“The discovery of new Li-ion conductors has largely proceeded by extending known superionic compounds into new compositional spaces. In this paper we present systematically the attributes of compounds that lead to high Li-ion conductivity, thereby developing specific criteria by which to look for better conductors.”
And now, I will translate into normal human speak.
“Previously, we were clueless, and just mucked around with stuff. Now we are not.”
Abstract is:
Lithium solid electrolytes can potentially address two key limitations of the organic electrolytes used in today’s lithium-ion
batteries, namely, their flammability and limited electrochemical stability. However, achieving a Li + conductivity in the solid
state comparable to existing liquid electrolytes (>1 mS cm −1 ) is particularly challenging. In this work, we reveal a fundamental
relationship between anion packing and ionic transport in fast Li-conducting materials and expose the desirable structural
attributes of good Li-ion conductors. We find that an underlying body-centred cubic-like anion framework, which allows direct
Li hops between adjacent tetrahedral sites, is most desirable for achieving high ionic conductivity, and that indeed this anion
arrangement is present in several known fast Li-conducting materials and other fast ion conductors. These findings provide
important insight towards the understanding of ionic transport in Li-ion conductors and serve as design principles for future
discovery and design of improved electrolytes for Li-ion batteries.
Hence, solid state **is** the state of the art. It’s just not as current-capable as liquid electrolyte.
A manufacturer investing in something with no real business model? Riiiggghhhtttt
Want long life? LiFePO4 batteries. Not picky about charging like common LiPo or LiION can handle a significant more charge cycles and are way more durable.
You can even abuse them by leaving them in a charge cycle for days without damage.
Definitely LiFePo4. We have products designed around them. Sure they cost more, but they last longer and you can use pretty dumb chargers attached to solar cells to charge them. A lot of solar powered garden lights that are higher quality use them. 2 dollar Wally world solar lights still use NiCad.
I also started buying AA and AAA NiMH batteries about 5 years ago and I have yet to wear one out. Hundreds of charges.
Solid electrolytes will still suffer from dendrite growth, eventually leading to failure. There are also issues of piezoelectric effect, magnetostriction, and thermal effects, all which cause strain in the battery, which will contribute to dendrite, cracking, fatigue, and failure.
Look at how large scale power cables fail, with “water trees”. It’s not even well understood how and why it happens, but it does.
” as liquid electrolytes are the main reason batteries catch on fire.”
Lithium is what makes lithium batteries catch fire. The electrolyte is flammable, but cannot burn or catch fire spontaneously since there’s no oxygen and no ignition source inside the battery, and mere exposure to air by a broken cell won’t do it.
The lithium on the other hand is capable of spontaneous ignition when the battery is damaged, and once you get it going it will burn in just about anything that contains oxygen, including water and CO2. The energy released from the chemical combustion of lithium in the battery is what evaporates the electrolyte and spews it out, where it makes the nice big flames.
A battery with solid state electrolyte such as this is not “inflammable”. It’s just a question of getting it started. Lithium, once ignited, will burn much like a strip of magnesium, and there’s also sulfur and other easily burning chemicals inside the battery besides the electrolyte.
“inflammable” was supposed to be “unflammable”
What the heck is “exponentially longer”? The passage of time waiting for your girl friend to get ready to go out? I know. You are all thinking e to the x for x greater than 1 so it depends on the length of x. But I think the author may have something else in mind.
Watch the chemistry be doped to shorten service life for profits.
In my experience, standard lithium ion batteries keep on working as long as you don’t overcharge or overdischarge them, they just increase in internal resistance to the point where they no longer work well in the original application. The oldest one in my collection, from a really old analog cell phone, is about 16 years old now and still works well in a LED flashlight.
The lithium battery wear mechanism causes lithium to undergo unwanted reactions and form compounds which lock the lithium away from use, and the capacity of the battery degrades over time.
Here’s what will (probably) happen if this becomes reality:
They’ll just make the batteries of their phones so small that they last no more than 1-2 years and you are forced to replace your phone. At least it puts an end to the problem that lots of people are still using old phones with security issues that are known for many years and won’t be fixed.
How is a security issue unfixed for years any worse than security issue unfixed for a few weeks?
Folks, Just remember the 3 choices we engineers have to make:
+Good
+Fast
+Cheap
We can ONLY choose 2.
Good and fast? It’s Expensive
Fast and cheap? It will be crappy
Cheap and Good? It will be slow as…waiting for the perfect mate.
Okay, so let’s wipe down the whiteboard/blackboard. Bottom line is If we want it good, fast and cheap? We will have 2 options.
*The Fabrication process, will take longer then normal/forever since we have to grow everything perfectly, merge everything perfectly and perform an insane amount of tests to make sure it gives what we made it to do.
*The Acceptance process, we will spend a retard amount of money to pick out our perfect PR agent, Marketing campaign, Political Action Committee (PAC) to bullshit that we have the best tech, prepare to wine, dine, and court our repesentatives with high class “escort ladies”, high end environment and stims AND proper electoral contributions for the DoD and DoE counsel reps.
-=-=-=-=-=-=-=-
Quick segway, derail. The boss gives you a task on a solid alternative on how to do things “better”. You bust ass, you test proof concept, you create a 50 pg report on how this is the most “optimum” solution. Long story short you are the sucker, he has been playing golf with the rep of the big company (with the acceptance process listed above). And your “hard work/research”? Yeah, that was just to throw it to the higher ups that your bastard piece of shit boss needed to provide at-least 1 alternative to the choice he already selected before even assigning the task to you.
“Get Shwiffty, dump one his dee-sssk,
yeeee-ah, once a day,
before you become Brian Nicko-ols”
Open access to the article:
http://s3.amazonaws.com/mavrl-web/publications/40042e0f-777d-35f7-95ca-9312478cc0cb.pdf