Have you ever seen the science experiment (or magic trick?) where you get water supercooled to where it isn’t frozen, but then it freezes when you touch it, pour it, or otherwise disturb it? Apparently, ice crystals form around impurities or disturbances in the liquid and then “spread.” A similar effect can occur in metals where the molten metal cools in such a way that it stays melted even below the temperature that would usually cause it to melt.
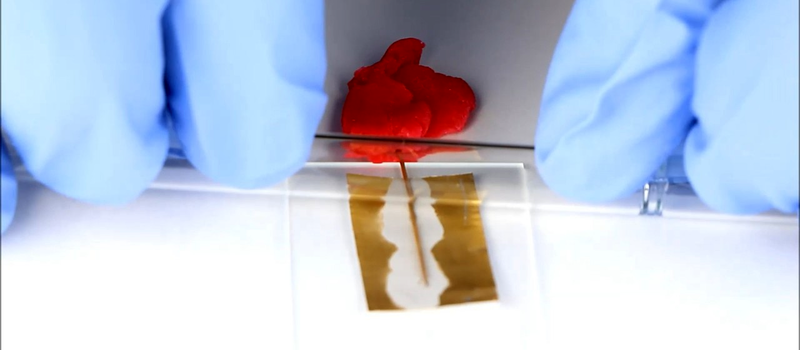
[Martin Thuo] at Iowa University used this property to make solder joints at room temperature using Field’s metal (a combination of bismuth, indium, and tin). The key is a process that coats the molten metal with several nanolayers that protect it from solidifying until something disturbs the protective layer.
Researchers hope to create room-temperature soldering using this technique. The biggest problem so far is that Field’s metal will melt again at 62 degrees Celcius and that makes it impractical for many electronic circuits that can see temperatures higher than that.
The article in Nature has a few videos you can check out. If you haven’t seen the supercooling effect in water before, you might enjoy the video below.
Thanks to [Alex Rich] for the tip.















Sounds cool, but I’ll believe it when I see it in the store. (Which I hope to.)
Iowa University? Come on…
UofI verses ISU — only a hundred or so miles different…
I was confused why we need cooler soldering.
“hot soldering techniques can sometimes damage the materials used in flexible electronic devices, or the ever-smaller parts on today’s microchips.”
So not a pointless field of research, neato.
“pointless research” is an oxymoron, we might not know the “point” (translated as practical use) at the current time but by its very nature research needs to be done before we could ever know if it had a “point” or not.
62C is a little low to be useful. That limits a lot of applications.
This stuff seems to be like chip-quik
Wasn’t there an article on Hackaday about a similar glue? It’s liquid inside a tiny bubble, which when the bubble is broken, the liquid comes out.
Yep, basically http://hackaday.com/2015/11/15/powdered-glue-sticks-when-squished/ but for metal/soldering.
How stable are those magic nanocoated supercooled liquid beans? Any random cosmic ray that comes along could trip them into solidifying at any time.
You can’t form a good solder joint with this. At such low temperature, your based metal and the solder doesn’t form a bond – i.e. you’ll get colder solder joint that can peel off easily. The understanding how a solder joint works is essentially to these kind of research. (pdf link below)
http://www.asminternational.org/documents/10192/1849770/05338G_Sample_eBook.pdf/8dd737af-b574-45b8-ab4f-ea2e5843f8e7
>The bond between solder and base metal is more than adhesion or mechanical attachment, although these do contribute to bond strength. Rather, the essential feature of a soldered joint is that a metallurgical bond is produced at the filler- metal/base- metal interface. The solder reacts with a small amount of the base metal and wets the metal by forming intermetallic compounds. Upon solidification, the joint is held together by the same attraction between adjacent atoms that holds a piece of solid metal together.
aka Van der Walls forces.
Incidentally the same as the forces that bond graphene layers in pyrolytic graphite together.
Interesting research though, have a few ideas for improvements.
You out a word there in Iowa State University. There is no Iowa University and the University of Iowa isn’t nearly cool enough to pull this off. Very interesting tech. 62 C does seem pretty limiting but there could be some amazing uses for this.
As long as it actually makes a good metallurgical solder joint, I think it would be pretty cool to put these in place of the solder balls on a BGA. All you’d have to do is press the BGA chip down onto the board and you could get an instant 600-point connection.
But thinking about it, its not really that much easier than just placing the BGA and reflowing it, Given how tricky BGAs can be from a metallurgical perspective, I doubt you could scale this up to anything beyond a few hand-assembled prototypes.
Why does that video narrator sound like a robot, is that TTS?
So, is this better or cheaper than using some kind of conductive expoxy and just glue the component on the board? And if glue does not cut it for your application, and you have a problem with the high solder temperatures needed for tin-copper alloys used in todays RoHS processes, could you not just make a ‘better’ alloy with lower melting temperatures, without jumping on the leaded solder train again?
Chip Quik does the same with the Sn42/Bi57.6/Ag0.4 paste they sell, and it melts at 138°C…