Battery technology is a constant chemical war between the laws of physics and the desire of engineers to make devices smaller. On one side, the laws of physics declare that there are limits to how much energy you can store inside a battery, and on the other side are the engineers looking for ways to sneak around these laws. For many devices, the best compromise between these two sides is the lithium ion battery, usually abbreviated to Li-ion.
Chemistry
As the name suggests, the Li-ion battery is based around the chemistry of lithium. The least reactive of the alkali metals, lithium atoms are small and light, with an electron that is just itching to leave, forming a positive ion. This electron with wanderlust also gives them high electrode potential, meaning that an lithium infused electrode can create a relatively high voltage: about three volts.
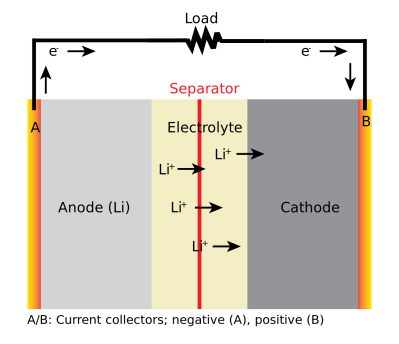
A Li-ion battery is composed of two electrodes with an electrolyte between them. For most, the negative electrode is made of graphite, while the positive electrode is coated with lithium in the form of a lithium oxide, usually combined with phosphate, manganese or cobalt. The electrolyte between them is usually an organic solvent, with a lithium salt dissolved in it to create plenty of lithium ions floating between the two electrodes. These two electrodes are also separated by a permeable layer that allows the ions through but keeps the electrodes from touching each other.
When the battery is charged, the lithium ions move into the negative electrode (called the anode) becoming trapped in the crystal structure of the graphite. As the battery discharges, the ions move back towards the positive electrode (the cathode), creating the electrical energy flow. Charge the battery up, and the ions flow back into the graphite. This process is quite efficient: you usually get out between 80 and 90 percent of the energy that you put in released as electrical energy.
http://www.youtube.com/watch?v=QNWpNlcmB1w
These advantages have meant that Li-ion batteries have become the preferred type for many modern devices, from smartphones to electric cars. In fact, many of these devices will use exactly the same batteries: take apart the batteries of a Tesla electric car, and you’ll find hundreds of the same 18650 batteries used in devices like portable batteries and e-cigarettes.
Danger, Danger!
So, the batteries are light, efficient, and can hold a lot of energy. Why aren’t they used everywhere? The problem is that Li-ion batteries are, in effect, a chemical fire waiting to happen.
First, this chemistry needs to be carefully controlled. If you overcharge a Li-ion battery, they can explode. To prevent this, most Li-ion batteries are fitted with controllers that limit the flow of energy in and out. When the charge reaches a certain level, this controller stops the flow of energy, shutting down the battery or the charging process.
Second, if a Li-ion battery is punctured, or has a mechanical failure that compromises the separation between the two electrodes, the two terminals become short circuited, heating the battery and releasing the lithium. This starts to react, reaching a temperature of up to 500 C. This melts the other materials in the battery, exposing more lithium, which heats up, causing a cascading reaction. In other words, the battery catches fire and burns itself up.
These problems are multiplied when Li-ion batteries are being stored or shipped. Imagine a warehouse full of these batteries, or an airplane hold full of batteries being shipped. If a single battery fails, or something else catches fire and exposes these batteries to high temperatures, you have a problem.
The batteries are designed to prevent this from happening by sealing the chemistry in a tough polymer pouch that is hard to tear, which also allows the batteries to expand slightly as they charge. (Emphasis on “slightly”. The battery in the headline is past the end of its life.) This also gives rise to the name “lithium polymer” that is used for some batteries, especially those used in drones and RC models that need to stand up to rough treatment.
http://www.youtube.com/watch?v=BLc74Qpvweg
This is also why Li-ion batteries are sometimes difficult to ship. Airlines have stopped accepting them as cargo on passenger flights, and UPS and FedEx have introduced new rules about how loose batteries must be packed for shipping. Any package that contains more than eight batteries also has to be specially marked as dangerous cargo.
Most airlines don’t restrict carrying Li-ion batteries in carry-on baggage, as long as they are fairly small. United Airlines, for instance, allows batteries with capacities of up to 100 watt hours inside a device, or two additional batteries that are properly packed to prevent short circuits. The TSA allows small batteries installed in devices in checked baggage, but restricts loose Li-ion batteries. So, if you are taking a prototype or spare batteries on a trip, best to remove the batteries and carry them on with you.
















one thing that needs to be said; there seem to be 2 classes of cheap hobby lipo batteries. people informally call them ‘air’ and ‘land’ batteries.
for flying copter use, it would be Bad(tm) for it to fall from the sky when the BATTERY decides its time to shut down. so, they don’t have the protection cutoff (LVC, low voltage cutoff) in the battery and, instead, they put it in the copter. the copter will blink its lights AS it gets to the lower limit and the pilot on the ground can gracefully land the craft.
otoh, land based rc toys won’t fall from the sky and its less urgent that the operator stops the toy before the battery exhausts. in that case, they put the protection circuit in the battery.
people often don’t know this. it gets worse when people re-use air batteries for DIY project (I admit to doing this; before I knew better!) and they run them down to below the LVC. they usually have no idea what they are doing is unsafe, either.
Did not know that. Thanks!
Laptop batteries also don’t have any protection at cell-level, all protection is on PCB inside battery. So when using such salvaged 18650 cells it is wise to add protection circuit to prevent overdischarge. Luckily there are cheap charger+protection boards on the eBay so it’s easy and cheap to integrate them in DIY projects.
Interesting, I’ve stripped broken parts of these batteries before but I haven’t had occasion to use them, but in case I do, is there an issue with adding a protection circuit on top of a battery that may already have a protection circuit?
I guess I’m saying if I find 18650’s in one of my junk drawers, and I can’t remember where I took it from (laptop, battery charger, etc..) can I add a protection circuit anyway or will it present new issues, in the case of the battery already has the protection circuit built in, like voltage drop or current limiting (past what is intended)?
Ummm wut? My ESC’s on all my ‘land” RC’s have the protection circuitry built into them.,,,
The protection circuit (PCB) is not built into the cell itself. Build inside of the cell is stuff like the CID and other mechanical elements which help the battery vent rather than explode. When we are talking about a protected cell, we are talking about converting the cell into a battery, or an all-encompassing unit that can be safely used by a consumer. A cell without protection should not be used by the average person because of the safety risks.
If you are salvaging 18650 batteries from used laptop packs, there are a few things to consider. Usually, those cells are low quality (low amps, low capacity). If they are old there will be voltage variations between the cells. Every voltage variation between a cell, after the cells are connected into a battery, will create further degradation as voltage is pulled abnormally from cells to make up for discrepancies.
When talking about protection we are usually talking about a small PCB attached to a single cell, for use in a flashlight for example. A BMS is usually used when connecting 2 or more batteries together and provides the same amount of protection (overcharge, overdischarge, short-circuit, etc.) as well as power balancing.
You can tell if a cell has protection easily by looking at the bottom terminal of the cell. If it is something other than steel (usually copper), has holes, moves a bit, or anything like that it is most likely a circuit board. The second thing you can do is look on the cell for a wire that runs under the PVC from the negative to the positive terminal. This won’t be on unprotected cells, but are required for protection.
“On one side, the laws of physics declare that there are limits to how much energy you can store inside a battery, and on the other side are the engineers looking for ways to sneak around these laws.”
Which law is that? I know, why don’t you identify the energy density of this and the other popular battery technologies so that this statement can be quantified?
No details on the differences between the Lithium battery chemistries? Which one is amorphous? Which one has the slowest self degradation over time? Which one is constructed in a jelly-roll fashion? What is it about the LiFeSO4 batteries that makes them a useful replacement for SLA batteries?
No info on the self degredation based on the storage temperature and level of charge, yet you talk about arbitrary US TSA and airline policies?
No info on cold temperature performance as compared to Alkaline, and the others?
No info on hot temperature performance? If you’ve left a cell phone in the sun you have already experienced the result?
No links to the battery management system ICs that get packaged in shrink wrapped battery packs to perform the over charge, over discharge, short circuit and over voltage protection?
Why do the batteries swell? By how much?
Nothing on charge balancing cells?
Not to mention that it’s not the lithium in the battery that burns, but the electrolyte which is a hydrocarbon based solvent. It’s comparable in energy to stuff like acetone or gasoline. The reaction between lithium and oxygen and water in the air generates heat, which vaporizes the electrolyte, which creates the billowing cloud of smoke – and if you give it a source of ignition it will also make a bunch of flames. The battery may or may not get hot enough for autoignition, but if it does then the reaction is just going to be that much faster.
Internal shorts in the battery quickly burn out by simply melting whatever is closing the circuit.
While source of ignition might be the battery itself. Punctured batteries often tend to not only smoke but also catch fire. Because, you know, where there’s smoke, there’s fire…literally in this case.
If you asked google, you’d already have the answers, and a pastrami sandwich*.
*sandwich.google.com in beta, may not be available in all dimensions.
Thanks Jake,
Are you an author for Hackaday too?
Apparently not. And sadly, still none after your comment. Rather than merely show your superior knowledge / that the author didn’t write a full article, would you care to share it with the rest of us? Whilst I’m glad you’ve given me a list of things to google, I can’t help but feel it’d have been more in the spirit of HAD for you to share your knowledge – perhaps a follow-up article, given the quantity of stuff you’ve got. Would be much appreciated.
100 what hours you can get 220 watt hours from the 4 ah 56 volt ego lawn mower battery
http://www.google.com/search?q=ego+56+volt+battery+specs&safe=strict&hl=en&gbv=1&sout=1&prmd=ivns&source=lnms&sa=X&ved=0ahUKEwjM_IiFnunMAhXIFh4KHdrFDRwQ_AUIBA
try to overnight one from ebay and see what happens
“Cathode: Charge Departs” or CCD. Nice, easy mnemonic to keep anode and cathode straight. Electorns leave via the cathode (-) and return via the anode (+).
Comment system needs work, HaD… WordPress…
Richard, you have Anode and Cathode backwards. Electrons depart via the cathode and return via the anode. “CCD” is the mnemonic: Cathode: Charge Departs.
According to Wikipedia (https://en.wikipedia.org/wiki/Cathode), it is where conventional current departs – i.e. it is where electrons return. So technically a battery has positive and negative terminals, but the cathode and anode aren’t fixed – when it is discharging the cathode is positive, but when it is charging the cathode is negative. Also repeated here: https://en.wikipedia.org/wiki/Lithium-ion_battery#Construction So technically the two parts shouldn’t be called the “anode” and “cathode”, because they’re both depending on the action. Further down that page (“Battery Life” -> “Degradation” -> “Anode”), there is a comment about the terminology as applied to Lithium cells: “There is a historical note about the terminology of Anode when referring to Lithium cells, which started as Primary (single discharge) as exampled with Lithium Thionyl Chloride cells. The Anode is classically the electrode where Oxidation is taking place in electrochemistry. This is true on discharge but with a rechargeable system the electrode switches back and forth from anode to cathode with cycling. The less ambiguous term for secondary cells electrodes are Positive ( anode on charge, not discharge) and Negative. This is the polarity measured on any cell with a volt meter.” Therefore this article is correct.
Interesting and thank you for clarifying the terminology here.
you’re doing gods work here, friend. well done! Keep beating back the demons of ambiguity and terminology misuse in science.
This is a common mistake for folks who don’t know the whole picture. I tell my students “a cathode adds electrons to the internal widget”. A CRT- the cathode is where the electrons pop off of, inside the device. Same for a battery (except the electrons reduce a chemical.
they keep letting these things on planes…
yet you’d get arrested for even attempting to bring any other kind of simillar-but-less-dangerous substance onto a plane… why?
does this have anything to do with that “mysteriously” burnt hole in an aircraft?
i doubt it was intentional, but seriously, TSA would get jailed if they let turpintine or acetone on the plane, which can be easily put out with a regular fire-extinguisher and would NOT put a hole in metal.
so why is the more dangerous substance allowed? no, really, why?
im asking a hard question, does that make me bad?
am i just bad cuz im hatin on the thing you need in order to access facebook?
do things like facebook override safety and the lives of very real real-life people???
My mother works in aviation screening, and frequently has arguments with passengers over what can and cannot be brought onto a plane or what needs to be placed in a tray for scanning.
One example being the metal detector, she’ll ask people to put metal items in the tray then walk through the scanner. They do so and the scanner goes berserk. Why? The mobile phone in their pocket. When pointed out, the response is often, “Ohh no, the phone is plastic!”
Those same passengers, blissfully unaware that the phone contains metal parts, also contain other dangerous components.
Now put yourself in the shoes of the aviation screener, and try to explain to that non-technical person why they can’t bring their smartphone/laptop/portable music player, on the aircraft.
Can’t bring their smartphone/laptop/portable music player on the aircraft? Since when?
When I last flew in a plane, you couldn’t operate those things while the plane was taking off, sometimes not even in flight either. But in those days, laptops cost a small fortune and phones were neither smart nor all that portable. (That was in 1994. GSM was the “new kid on the block”.)
However, if they considered NewCommentor1283‘s remarks, they could well be banned.
But more relevant to the discussion is that airlines specifically require you to carry lithium batteries in carry on baggage.
I might be off by a couple years, but as far as I recall, in 1994 laptops and the few phones around all used NiMh cells.
“and try to explain to that non-technical person why they can’t bring their smartphone/laptop/portable music player, on the aircraft.”
Only to be counted by someone holding up a printout from their own website saying that specific policy is that lithium cells MUST be in carry on baggage. I had just this discussion with an aviation screener when I moved overseas. No one would ship lithium batteries, and the airline required them not to be checked. No problem I put 10x 2.4Ah 3cell batteries in my carry on. The aviation screeners flipped and then got super defensive when I showed them the requirements were that they be on my carryon on the website of the airline company AND on the website of the airport.
Are you okay man? Take a chill pill.
And Facebook sucks, I think. I don’t use it because it sucks.
Maybe a hard question, but the answer is easy: people would stop flying if they couldn’t take their smartphone and laptop with them. I travel on business, but would not be able to do so if I couldn’t bring my Li-ion powered devices, in particular my laptop and video cameras. I simply wouldn’t be able to do my job. I’m guessing that would be true for most business travelers, and most holiday travelers would find an alternative transportation mode if lithium batteries were banned.
What accident involved a hole burnt in an aircraft, and from what facts are you implying that this was caused by a lithum battery?
Why? Because logic and sense doesn’t get your agency increased funding.
All batteries inside commercial devices have safety measures to reduce fire and explosion risk to minimum. Even in case of complete safety failure they can’t seriously endanger plane. If laptop catches fire in the passenger part of the plane it would be quickly put out. Batteries in cargo part are more dangerous because there is no one there to quickly stop fire from spreading and crashing the plane. Using them as a bomb is not realistic scenario, they can catch fire, they can explode, but damage to surrounding objects is not really a considerable threat.
No one requires a battery to have a safety measure, and you most definitely can just throw in a bunch of lithium cells unprotected in your carryon. The rules recently changed to say no more than 2 batteries, but the requirement is still less than 160Wh per battery. That is a HUGE frigging lithium pack.
“does this have anything to do with that “mysteriously” burnt hole in an aircraft?
i doubt it was intentional, but seriously, TSA would get jailed if they let turpintine or acetone on the plane, which can be easily put out with a regular fire-extinguisher and would NOT put a hole in metal.”
Not at all. A lithium cell is a fairly shortly lived firework. It doesn’t have the temperature or duration to burn through an aircraft even if sitting on the damn hull when set off. It may cause a small localised burn and injure someone but that’s about it. Now on the flip side 1L of turpentine set alight can quite easily kill a whole group of people in a big firely explosion.
“which can be easily put out with a regular fire-extinguisher”
Not sure why you make this comment. Fire extinguishers on aircraft are Halon 1211 and have equal capability to put out lithium and hydrocarbon fires. Both suffer from problems of spontaneous re-ignition.
“so why is the more dangerous substance allowed? no, really, why?
im asking a hard question, does that make me bad?”
No you’re not asking a hard question. You’re asking an ignorant question. The more dangerous substance has been disallowed.
There’s also the “people are idiots” factor, if you make the rules “better” for the 1% of the population who care that much, you make the rules much more complex for the other 99% to understand and comply with, and make the kerfuffle at the airport even worse.
I hate the current airport security theatre as much as the next guy but you’ve just got to relax, accept it as a necessary evil and comply with it – the guys at the security desk have no more say than you do, and it’s not the place to start an argument unless you really want to end up on some sort of list.
If you hate it, bitch to your local politician about it, join Liberty or EFF or something.
“When the battery is charged, the lithium ions move into the negative electrode (called the anode) ”
– I am not a battery expert by any means, but is the Anode really the negative electrode?
Yes, because this is a power source rather than sink. The anode provides electrons, the cathode receives them – hence a source of power (like a Li-Ion cell as discussed here, but any kind is the same) has a negative anode while something that uses power (say a diode) has a positive anode.
Another new chemistry that’s available now: http://www.ev-power.eu/LTO-technology/LTO1865-Rechargeable-Cell-2-4V-1300-mAh-Lithium-Titanate.html
Difficult to say yet if the claims of safety and better low-temperature operation are true. Energy density is about 30% of that of normal li-ion.