Rocket engines are undeniably cool. Experiencing the roar, seeing the fire, and watching the rocket blast off into the sky… what else can you ask for? Well, for [NightHawkInLight], a transparent rocket body is the answer.
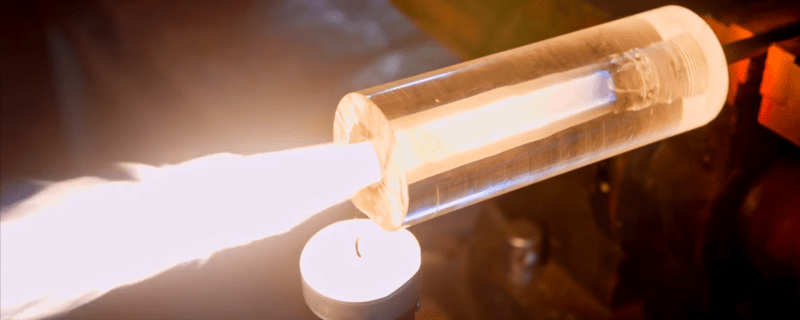
Based on previous work by [Applied Science], he uses an acrylic rod as the rocket body and as the fuel. Bring a flame into the acrylic, apply oxygen from a canister at the other end of the body and voilà! The rocket engine starts nicely, and even better, the intensity of the burn can be controlled via the amount of oxygen provided.
The construction is straightforward, just drill a hole in the acrylic rod, tap a thread at one end, and attach a brake line that goes to the oxygen canister. As he explains, in solid rocket engines the fuel and oxidizer are mixed together. A typical recipe in amateur rocketry, known as rocket candy, is a mixture of sugar (as the fuel) and potassium nitrate (as the oxidizer). In a hybrid rocket engine like this, the fuel and the oxidizer are separated from each other and they only mix in the combustion chamber. In this case, the combustion chamber is the fuel itself!
While this rocket engine may not very efficient nor produce a large thrust, it is definitely a great attention-getter. [NightHawkInLight] did a great job with this video as it has great educational value for those interested in rocketry. Other ways of building rocket engines at home include this PVC pipe version, or even a 3D printed one.















Actually the red fish has more memory than the popular idea.
http://hackaday.com/2012/09/26/hybrid-rocket-engine-uses-acrylic-as-fuel/
The average HaD blogger is either too lazy for search back, or considers its audience as a kind of volatile memory.
Try clicking on the second link in the article and see where it leads you.
And don’t be so mean. :(
Mr. Elliot be like >> http://m.memegen.com/lg2eox.jpg
http://static1.fjcdn.com/thumbnails/comments/How+determined+are+you+to+get+coloured+text+_34d2a55570344c61a53bddb82226f125.png
Because I’m an ornery cuss, I wanna try it backwards and shoot fuel down an oxidiser.
Scary but interesting concept.
Maybe some sort of air stone [ fish tank type ] may make it a little more stable.
Just realised that squirting molten plastics through PTFE might fit that definition…. Then you’ve got a 3D rocket etcher.
I would not count PTFE as an oxidizer, on the contrary, it is sometimes used as a fuel. Probably because it does not melt. But it produces nasty/dangerous fluorine compounds when it burns.
Why not using any type of oil as the fuel in the reverse hybrid concept?
Ah, yah several sources refer to it as an “oxidiser” when it’s really a “fluoridiser”, which is particularly exothermic when metals are used as fuel but may not work with other fuels.
I was making a haha only serious point about the dangers of thermal runaway in 3D printer hotends, but it might only apply to metal filled filaments, and you still should have a couple of hundred degrees safety margin, much like frying in oil is safe enough with precautions.
You want to build a BBQ grill?
I admit, I’ve been reading HaD, at least once a day, for many years. I wonder the average length of time HaD followers have been revisiting. Perhaps the previous post was deemed older than the average HaD demographic. Although I’d like to think the sort of enquiring mind that would frequent a site like this one, wouldn’t have much hesitation in looking through the old archive posts. (:
Cool, I wonder if this could be built into a solid fuel scramjet, hey if your not going to space the oxidizer is free.
How about a sci-fi disaster movie where they drilled a hole through a combustible asteroid with the planetary defense laser, unsuccessfully trying to deflect it, then when it hit atmo it accelerated….
That sounds hilarious, though perhaps a bit dark. :D
Though it need not be, maybe it turns a tangential surface hit into a miss.
Better dead than to look bad, right?
“Houston, we have a problem!”
Combustible Asteroid!!, tonight on SyFy.
Combustible Asteroid!! We’re all getting coal for Christmas! Coming Dec. 25th!
I’m not sure how we’ll defend against it though.
Bruce Willis died deflecting the last asteroid…..
Another strange thought… what if you rolled a newpaper up tight and jammed it in a chunk of fence post (Steel tube like for chainlink) and applied compressed air… useful torch or burner ???
That’s a torch! It’s called a thermal lance: https://en.wikipedia.org/wiki/Thermal_lance
I’ve seen them used for demolition of steel structures, where you want to elevate workers in a platform above the structure.
If you hook an oxygen tank to steel brake line you can make your own thermal lame for small projects.
A bit of steel wool stuffed in the end serves as an ignitor while the line gets up to temp.
*thermal lance, thanks android
I was aiming for a tad less heat than burning the steel.
Another thought, waste oil soaked toilet paper in a can blown by a leafblower for de-icing duty???
Blower fed glasspack muffler with kerosene feed??
An oil soaked roll of toilet paper at least makes a good fire starter. HowI know? A rat or mouse bit holes into a bottle of lawn mower oil in the shed and the oil soaked a roll of paper. Instead of throwing it away I just thought about a good use for it. :-)
There was a hybrid engine invented a while ago that used synthetic rubber as fuel, with a gaseous oxidiser (nitrous oxide IIRC). Had the simplicity advantage of a solid rocket, with the throttling and control ability of a liquid fuelled rocket. Particularly the ability to switch it off, which solids can’t do. It gave enough thrust to be practical. Can’t remember where I saw it, now.
Bit of Googling tells me it’s Spaceship One’s engine. Great idea, can’t believe NASA never thought of it.
Nasa thought of it, rejected it for practical/political reasons
Political possibly meaning “We can’t have success with a nickel and dime motor when we’ve spent billions on propulsion research, we’d have our budget quartered.”
Now what do we do with all these used tires…
…let’s burn ’em to get to Mars!
The practical reason is that it’s a bloody dangerous design.
When you turn it off, the residual heat in the engine melts the rubber, which clogs the nozzle, and if you try to turn it back on it will blow up. That’s actually a generic problem for hybrid rockets, and it applies for running the engine too slowly as well, which limits the throttle range. If the fuel breaks up due to vibration, or it melts and starts to run, the engine explodes.
So it can’t actually be re-started, and can’t be throttled much below full power once it’s really going, or else you have to stop it entirely. The only real advantage over a solid fuel booster is the fact that you have an “abort” button – but then you still have the problem of a huge oxidizer tank in free-fall to somewhere inconvenient.
Half way through reading Your comment I immediately thought of a solution. In side the nozzle have a sharp push rod that you can pneumatically push out past the tip. Coat it in Teflon or a non stick ceramic, and extend it out the nozzle when you shut down the engine. You could take it a few steps further and make it a drill bit instead, allowing you to drill through any blockage.
Alternatively you could have the combustion chamber spin at roughly 100 rpm immediately after shutdown to force any molten plastic/rubber onto the chamber walls till it solidifies. You could combine the spin idea with the push rod to ensure no blockage occurs,
i thught that the aluminum based hybrid rockets didnt show many of these issues, of course almost all serious hybrid rockets use some sort of wax or plastic.
Actually, the first hybrid rocket was the GIRD-9. It flew in Russia, 17th August 1933, near Moscow. Liquid oxygen was injected into a combustion chamber lined with gelled gasoline/benzene paste, The vehicle was co-designed by Sergei Korolev who eventually led the Soviet space program. The vehicle achieved an altitude of 400 metres.
Here is a video of the launch: https://www.youtube.com/watch?v=ZiUGZTdS1c4
I kind of want to try this now; Logically, a motor like this would be safer than a solid rocket candy-type DIY motor, and presumably you could get away with using one of the small NO2 cylinders (wouldn’t give you a long blast, but probably enough for a short boost on a model aircraft or something). On that note, could you get away with using, say, NO2 and butane or something to create a liquid fueled rocket motor?
I assume you meant to say nitrous oxide, N2O.
N2O is nice to use because it is self-pressurizing, to about 750 psi at room temperature. You’re definitely on a workable track with the idea of whipped-cream N2O chargers, see this commercial version, for one, or just google for micro hybrid rockets. These are apparently D- or E-class motors, depending on whether they take 8g or 16g N2O chargers. (Or maybe by “small NO2 cylinders” you meant like a 5-pound tank as used in automotive nitrous systems? For some size of model aircraft, of course.)
You could certainly make a N2O/butane liquid fuel rocket, but you’ll have to pressurize the butane to get it into the combustion chamber — at room temperature it’s only about 20 psi. For a micro rocket, turbomachinery is slightly impractical, so the most likely means to pressurize the fuel would be a CO2 cartridge. Conveniently, CO2 has almost identical characteristics as N2O, so you’ll have very similar pressures on both sides. Once you’ve accepted the complexity and weight penalty of a pressurization system, I don’t see an advantage to butane over RP-1 (or equivalent, e.g. diesel fuel) with the same pressurization system.
Liquid propane is a little better than butane (~100 psi), but that’s still pretty weak — I don’t think you’d want to use it without pressurization either. Supercritical methane or ethylene could be your answers, with critical pressures of 670 and 740 psi, respectively. Or maybe liquid ethane or acetylene, with vapor pressures of 550 and 650 psi at room temperature. But I don’t know of a source for these (or any other fuels) in cartridges comparable to the CO2 and N2O chargers, so you’d probably have to get any of those in a cylinder and fill your own tiny pressurized tanks from them.
Yeah, sorry; Meant N20. Interesting; I’ll be honest, I hadn’t really considered the room-tempreture pressures. Would it be possible to draw liquid fuel into the system using the flow of the N20 with a venturi, sort of like an airbrush? or would that not get enough fuel through? I was going to ask wether it’d be possible to use the N20 to pressurise the fuel supply, but I suspect that’d probably be a recipe for blowing everything up. Pressurising a liquid fuel supply sounds like the easier way to go, anyhow, although I could always poke my nose in round chemistry for some of the other options (uni student).
I might have to have a bit of an experiment, anyhow; Probably with a hybrid motor first, though, as it sounds like they’re a rather more proven setup.
I don’t think I’d try the venturi thing — bringing the fuel and oxidizer into intimate contact before you want them to burn is rarely helpful. But it seems like it could work. I’m not sure what fuel/oxidizer ratio you could manage this way, but it seems like it would be tremendously oxidizer-rich. (Not as big a problem as it may sound — given reasonable temperatures in the combustion chamber, all the N2O will decompose exothermically to N2 + 0.5 O2 ; even the excess N2O helps.)
But actually, using N2O to directly pressurize fuel should be perfectly feasible, as long as you use a rubber bladder in the fuel tank to separate them. (Obviously “rubber” means some elastomer that’s compatible with both N2O and your preferred fuel; I have no idea whether natural rubber qualifies.) This sort of gas-bladder pressurization system is used in some real rockets (typically pressurized with helium) — though I think it’s used less as a chemical barrier and more to ensure the gas remains at the “top” of the tank, and the liquid at the outlet, even in free-fall.
A bladder-pressurized system could actually makes a lot of sense — since you’re using N2O vapor (not liquid) to match the volume of the fuel tank, the effective reduction in oxidizer capacity is small. And unlike using a CO2/helium/etc. pressurization system, you’re not adding an extra pressure vessel — just a little extra plumbing to the tank you have. The only real downside is the more elaborate construction of the fuel tank with pressure bladder. But I’m not quite sure how to make it work with the N2O whipped-cream chargers — they only have one port (liquid or vapor, depending which way up), whereas you really want two ports, one at the top for vapor, and one at the bottom for liquid.
Oh, one other option I’ve heard of for pressurizing a rocket fuel tank — dry ice! Do your calculations carefully, fill your fuel tank leaving a carefully measured ullage volume, put a carefully measured quantity of dry ice in a tray at the top of the tank, seal it up, and the pressure will build to whatever number you calculated for. (Measure a bit wrong, and you’ve made a dry-ice bomb!) I don’t know of anyone who has actually done this, but it has been discussed in the context of sounding rockets — the group discussing it decided to go for direct helium pressurization instead, as that was a relatively common, well-understood system. I wouldn’t recommend that approach to anyone without lots of rocketry experience, but this discussion wouldn’t be complete without mentioning it…
You’re reinventing the carburetor here, try messing with one off a small weedwhacker.
OK, you already suggested Ethane/Ethene but I would strongly recommend against Acetylene, it is explosive on pressurization alone. That means you can not (safely) pressurize it to more than several bar (I have read values between 2 and 5).
Butane has a very low vapor pressure for a pressure fed engine. This would only be some kind of torch. Propane has 8,3bar @20°C but Ethane is still better: 38bar@20°C and it has more Hydrogen, is lighter.
Nice. I think that oxygen supply could be replaced with a small fan, and the thing would still work. With less thrust of course.
Neat as a “Look how neat this is!” sort of thing. Now put it inside a metal containment tube, fitted with a cap at the oxygen input and a high temperature nozzle at the outlet to get some usable thrust.
On a more practical note, & get some points with your better half why not use the oxygen feed for toast; Place bread on porous ceramic plate. feed oxygen through plate to infuse bread, ignite with spark/ laser? voila – instant toast.
Monash Unin had one of these in the late 90s,
One of the solid fuels tried was sausage.
It worked, but smelt horrible
Myth busters – Salami Rocket. :-)