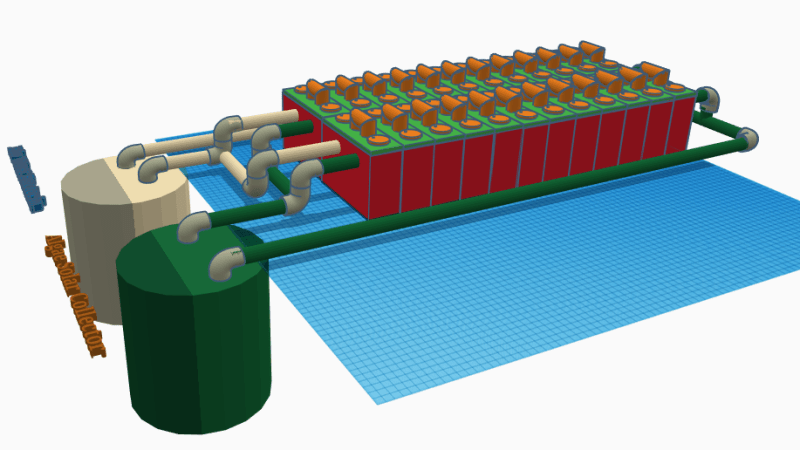
Battery cells work by chemical reactions, and the fascinating Hybrid Microbial Fuel Cell design by [Josh Starnes] is no different. True, batteries don’t normally contain life, but the process coughs up useful electrons all the same; 1.7 V per cell in [Josh]’s design, to be precise. His proof of concept consists of eight cells in parallel, enough to give his cell phone a charge via a DC-DC boost converter. He says it’s not known how long this can be expected to last before the voltage drops to an unusable level, but it works!

There are two complementary sides to each cell in [Josh]’s design. On the cathode side are phytoplankton; green micro algae that absorb carbon dioxide and sunlight. On the anode side are bacteria that break organic material (like food waste) into nitrates, and expel carbon dioxide. Version 2 of the design will incorporate a semi-permeable membrane between the cells that would allow oxygen and carbon dioxide to be exchanged while keeping the populations of micro-organisms separate; this would make the biological processes more complementary.
A battery consisting of 24 cells and a plumbing system to cycle and care for the algae and bacteria is the ultimate goal, and we hope [Josh] can get closer to that now that his project won a $1000 cash prize as one of the twenty finalists in the Power Harvesting Challenge portion of the Hackaday Prize. (Next up is the Human Computer Interface Challenge, just so you know.)


















Think of the microbes
There are none doing any work in these “cells” other than sitting in a galvanic zinc carbon cell so I think they are happy they have a home to live in for free.
The beginning of the MATRIX
Only due to the last minute change where we went from processors to batteries so the average person could relate. We are still the top of the food chain for parallel processing power. We suck as batteries.
Even the best lithium batteries have an energy density on the order of 1/50th the energy density of fat. The tradeoff is that the power density of fat is very low…
You’re ignoring the oxidizer / cathode in this situation, oxygen. Batteries contain an anode and cathode in the same package but for combustion we separate them to avoid catastrophe. If you have enough oxidizer to completely burn fat in the same place and sufficiently mixed you have considerable power density. All you have to do is kick it over the activation energy.
The power supply for the matrix was fundamentally flawed. It’s an interesting concept though, as human beings actually use animals in a similar way. If you think of modern farming practice where a cow / pig will lives in a shed and never sees the light of day, there are some real similarities. We feed the animal energetically expensive grains / silage which is turned into protein and fat in an extremely inefficient way. Much better to eat the grain / silage directly ????. I’m sure the matrix would have been better powered by standard renewables: wind / solar / hydro / nuclear.
Steak > efficiency
This is a zinc copper cell, there is no significant contribution from the microbes without a proton exchange membrane and high surface area carbon electrodes for them to grow on and exchange electrons with not to mention eliminate false readings from dissimilar metals oxidizing. https://youtu.be/vzm-QYQF3EQ He clearly states in this video there is a copper and zinc wire in the water. How did this win $1000 dollars?
Agree. Especially if anode has oxygen from microbes. (But next phase may not have algae in anode.)
I found reseach “Algae grown anode microbial fuel cell and its application in power generation and biosensor” (https://repository.hkbu.edu.hk/etd_oa/169#) that mentions about oxygen produced in anode by algae will compete to accept electron (If it is placed in anode, oxygen produced would accept electron in anode and reduce electricity generation of MFC.). That research used chemicals to alter mitochondrial behavior in algae in anode.
I wondered about this project log, showing a voltage reading from a pair of dissimilar wires sitting in a single tub of green algae water https://cdn.hackaday.io/images/3651011531363367340.jpeg
It’s one thing for hackaday to post links to projects that don’t actually work as advertised, but now they are giving them money?
Yeah, this is a copper-zinc cell with contaminated electrolyte. The microbes aren’t involved. I’ve done microbial fuel cells (Yeast, Methylene Blue, Dialysis tubing, Graphite for both electrodes). You get some energy out before the Methylene Blue kills off the yeast but not much.
Simple test: will it work with identical sized electrodes of graphite?
IIRC zinc bromine (aka ZCell, RedFlow etc) cells work like this, the zinc electrode is a complex porous plate of transition metals similar to how a Li-ion works.
Its got almost the same electrochemical potential as native zinc, and when depleted its voltage collapses down to near zero (part of the way these batteries can be stored fully discharged)
Ironically the very early NiFe cells would work perfectly now with 4 way monitors (voltage, internal resistance, cell pressure and pH) and would be equivalent to an early Li-ion without all the flammable side effects.
A good trick I invented is to make a “plate” of porous Bi which can be made mechanically or using BiMg (cough Roswell /cough) but this does actually work though the formation process is a bit tricky and needs a microwave oven!
I still can’t believe this was discovered in a garden shed with primitive equipment but it works very well indeed.
In other news:
– Beer-powered battery
– Urine-powered battery
– Dr Pepper-powered battery
– Blood-powered battery
– Sweat-powered battery
– Saliva-powered battery
– Diarrhea-powered battery
– cat foot-powered battery
In a zinc-copper cell nearly every fluid can be used as a (crappy) electrolyte.
Can I have my $8000 for my eight new innovative battery types, please? Thanks.
*food.
But a foot might also work…
Here you are: ???????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????? ….. spend it wisely!
Thanks for the post in the Blog, this is awesome! Of Course this was just the first version, the second version of the setup will use a permeable membrane, non reactive electrodes with Bacteria on the left, Plankton on the right.
https://youtu.be/YQyD5gFPOGw