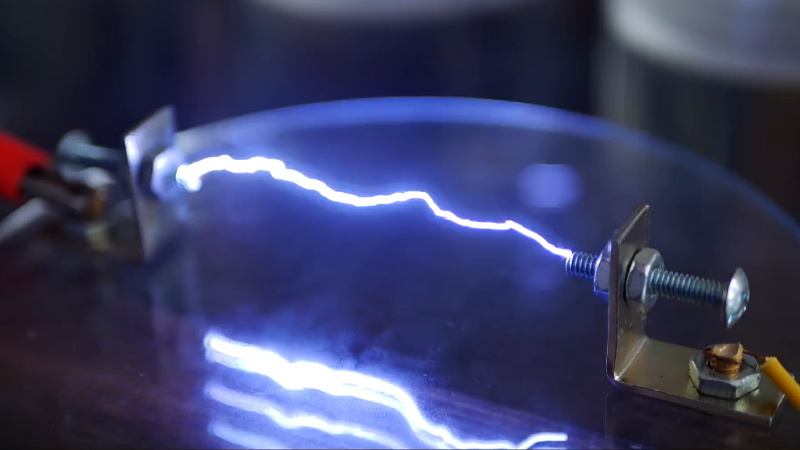
You’d be hard pressed to find a hacker or maker who doesn’t have a soft spot for the tantalizing buzz and snap of a high voltage spark gap, but it remains the sort of project that most of us don’t take on personally. There’s a perceived complexity in building a device capable of shooting a proper spark through several inches of open air, with connotations of exotic components and massive hand-wound coils. Plus, nobody wants to inadvertently singe off their eyebrows.
While the latest video from [Jay Bowles] might not assuage anyone’s fear of performing impromptu electrolysis, it does at least prove that you don’t need to have a laboratory full of gear to produce six figure voltages. In fact, you don’t even need much in the way of electronics: the key components of this DIY Marx generator are made with little more than water and some household items.
 This is made possible by the fact that the conductivity of water can be changed depending on what’s been dissolved into it. Straight tap water is a poor enough conductor that tubes of it can be used in place of high voltage resistors, while the addition of some salt and a plastic insulating layer makes for a rudimentary capacitor. You’ll still need wires to connect everything together and some bits of metal to serve as spark gaps, but nothing you won’t find lurking in the parts bin.
This is made possible by the fact that the conductivity of water can be changed depending on what’s been dissolved into it. Straight tap water is a poor enough conductor that tubes of it can be used in place of high voltage resistors, while the addition of some salt and a plastic insulating layer makes for a rudimentary capacitor. You’ll still need wires to connect everything together and some bits of metal to serve as spark gaps, but nothing you won’t find lurking in the parts bin.
Of course, water and a smattering of nails won’t spontaneously generate electricity. You need to give it a bit of a kick start, and for that [Jay] is using a 15,000 volt DC flyback power supply that looks like it may have been built with components salvaged from an old CRT television. While the flyback transformer alone could certainly generate some impressive sparks, this largely liquid Marx generator multiplies the input voltage to produce a serious light show.
We’re always glad to see a new video from the perennially jovial [Jay] come our way. While his projects might not always be practical in the strictest sense, they never fail to inspire a lively discussion about the fascinating applications of high voltage.















A salvaged neon transformer can provide 5 digit voltages at small amperages, providing a relatively safe spark gap experience. I found mine outside of a bar.
It’s also worth mentioning that nearly all neon transformers are internally shunted (at least the transformer-based varieties) to limit the output current, which means that the transformer can withstand a direct short of the output terminals without suffering damage to the secondary winding.
Negative resistance biyaaaatch
I remember the first NST i picked up off a dead sign, 15KV at 30mA..shocked myself so bad the first time I played with it that i went pail.
Then I made a “chicken stick” and life went on.
At a nearby mall the insulation was off the end of a neon light right in reach of most people. I use to grab it and then see which of my friends tried it. Most only did it once! They’d scream out in pain and be like didn’t that shock you, of course it did LOL. So of course once I got them with it, I’d have to resort to passing the voltage to them. Nobody would be near me near that light after that.
Did not know that Marx multipliers worked with AC input. I suppose if the caps can all charge during a half cycle, it orta work
The flyback transformer (LOPT) in the HV supply has internal capacitors and diodes configured as a rectifier and DC voltage triplet. DC output.
“Of course, water and a smattering of nails won’t spontaneously generate electricity.”
Though it might, this hasn’t got to the textbooks yet, developing field. In the presence of certain kinds of surfaces (Maybe zinc coated nails) water polarises or ionises, changes properties. There is charge separation. However if you pull electrons it will eventually deplete, however, there’s evidence that it can replace them by photon capture. Very intriguing stuff. Anyway, leveraging these properties might make for much enhanced water drop generators, true ways of massaging lightning out of water, like clouds do. This has drawn interest from ALL quarters, meaning there’s a lot of speculation in fringe fields about what it might mean, much like quantum theory, however, there’s real physics happening. This is a “straight from the horses mouth” presentation with included evidence, there’s not really a short way to get you fully up to speed…
https://www.youtube.com/watch?v=nSbg3cuZNRQ
“In the presence of certain kinds of surfaces (Maybe zinc coated nails) water polarises or ionises” water is a polar molecule. That is why it is a good solvent.
Yeah, but when it self ionises, it’s still all averagely neutral in bulk, this pulls negative ions one way and shoves positive the other. Without having to apply a potential to do so. Presumably in the past, electrochemists would see a few millivolts or microamps and figure they were getting some kind of junction, galvanic or Seebeck effect off the connections and wiggle things around, or blame stronger ions contaminating the water, maybe they rezeroed their instruments and forgot about it. However, knowing that peculiarities of water and surface structuring can cause charge movement, we can work on that and get more macroscopic effects.
Ahhh, Leyden jars.
From the title, I thought maybe somebody was finally trying to outdo Sir Humphrey Davy in building batteries of unusual size. After all, it’s been 200 years.
https://fineartamerica.com/featured/royal-institution-electric-battery-science-photo-library.html
BOUS’s? I don’t think they exist..
Z*A*P!!
I can smell the ozone just by looking at this.
Got to see the Z machine. They use water resistors too. Much bigger spark though!