When we see a CO2 laser cutter build around these parts, chances are pretty good that the focus will be on the mechatronics end, and that the actual laser will be purchased. So when we see a laser cutter project that starts with scratch-building the laser tube, we take notice.
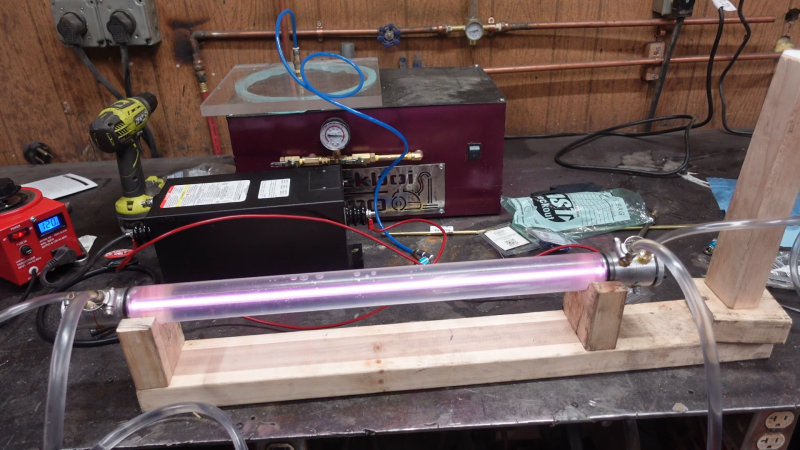
[Cranktown City]’s build style is refreshingly informal, but there’s a lot going on with this build that’s worth looking at — although it’s perhaps best to ignore the sourcing of glass tubing by cutting the ends off of an old fluorescent tube; there’s no mention of what became of the mercury vapor or liquid therein, but we’ll just assume it was disposed of safely. We’ll further assume that stealing nitrogen for the lasing gas mix from car tires was just prank, but we did like the rough-and-ready volumetric method for estimating the gas mix.
The video below shows the whole process of building and testing the tube. Initial tests were disappointing, but with a lot of tweaking and the addition of a much bigger neon sign transformer to power the tube, the familiar bluish-purple plasma made an appearance. Further fiddling with the mirrors revealed the least little bit of laser output — nowhere near enough to start cutting, but certainly on the path to the ultimate goal of building a laser cutter.
We appreciate [Cranktown City]’s unique approach to his builds; you may recall his abuse-powered drill bit index that we recently covered. We’re interested to see where this laser build goes, and we’ll be sure to keep you posted.















I was under the impression that the slightest trace of grease or dirt would cause the whole system to explode and/or catch fire.
real genius? that guy’s hands are scary looking.
You’re thinking of oxygen under pressure. This is CO2 [carbon that has already been burned] and nitrogen and often helium and sometimes water vapor (you can lase your exhaled breath).
No, you can get damaged optics though.
Yes, that was my thought: Why in the world does he touch these precious gold mirrors with his bare, dirty, greasy hands?
I wouldn’t call them precious. They are pretty cheap, but easy to damage. And it’s the wrong kind of mirror anyway.
The blue-purple gas pipe is not bad
i like this i needed it thanks
I’ve been interested in scratch building a co2 laser. But the water cooling aspect seems tricky and I’ve never worked with glass before. I know someone who could help though.
Given the standard method for disposing of fluro tubes in this part of the world is throw them in the bin and smash them cutting one up and releasing the gas is not realy an issue.
We’ll further assume that stealing nitrogen for the lasing gas mix from car tires was just prank…
Why?
Asking real questions here!
AFAIK the different chemical elements of air leak through tire rubber at different rates. Nitrogen is considered to leak slower due to larger size of its molecules, which makes tires being higher on nitrogen than the rest of the atmosphere. But i am not sure how big this difference can be. Probably depends how long it was since you’ve completely replaced air in your tires and how many times you topped up the pressure (given the loss was not caused by mechanical puncture).
Some places use nitrogen in the tires instead of air.
Tire shops here in Germany routinely fill tires with nitrogen. You can usually recognize nitrogen filled tires by the green valve stem caps they put on the nitrogen filled tires.
I don’t pay the premium (extra Euro or two) for nitrogen. I’m not running races, so the better performance at high temperature doesn’t matter.
I had the impression that the biggest thing that affected the stability of the tyre pressure was moisture content; no appreciably difference between 100% nitrogen and air (which is already 78% nitrogen) from a shop compressor where both are effectively dry. Only filling the tyre with air, direct from a portable compressor (so normal atmospheric humidity), that a difference might be noticed but only on vehicles and in environments where you might notice it (such as on a track).
Nitrogen is used in racecar tires because it reacts in a known way to the massive heat changes in a racing tire. It doesn’t necessarily give better performance than air; it gives predictable performance which is important when you are driving at the absolute limits of car and driver.
Regular air is about 80% nitrogen, 20% oxygen, and a small amount of water vapor, which is highly dependent on ambient temperature and humidity. The properties of how a tire performs when heating up changes with the water content, which can change on a day-to-day basis. It doesn’t change much, but at the extremes of racing it doesn’t need to change much.
Nitrogen is used in street vehicle cars because race cars do it so it must be better and the dealer said its better. For a street vehicle running well within legal limits, there is not a noticeable difference. Things like “leaks slower due to larger molecules” are bogus, in part because air is already 80% nitrogen, and in part because nitrogen molecules are smaller than oxygen molecules, the other major component (and smaller than CO2 molecules, which would be just as easy to use in tires).
Nitrogen was a dad/scam a few about 10 years back. They had this whole line they tried to feed you about how oxygen leaks out of tires faster and causes oxidation of the rubber from the inside. They said that in weeks to months the tires would go flat but it would last year’s with nitrogen. That was obviously bunk. Let’s say half the oxygen leaks out in a month. Well, air is around 80% nitrogen or so, give or take. You refill that O2. So now you have 12% oxygen and 88% nitrogen. In another month you have lost half the O2. You refill it again. So you have 7.2% O2 and 92.8% N2. After another month, You have 4.32% O2 and 95.68% N2. After another month, 2.6% O2 and 97.4% N2, after 5 months, 1.56% O2 and 98.44% N2, and after 6 months , 0.91% O2 and 99.09% Nitrogen. Whatever their claims as to the difference in between nitrogen and air, before long whether it be weeks or months, you would end up with almost 100% N2 because what they are describing is a “molecular drive”. It’s total BS. On top of that, they are not using a tank of nitrogen to fill your tires. They have a machine that makes it, and usually does not do a particularly good job. It usually only gets it between 90 and 95%, if it’s working right, but since no one actually checks what they put in the tires, it’s probably not working right. Total scam.
This is good
Good hacks break laws, challenge norms.
Use ‘old-school’ laser-gas mixture!
Propane torch running a good hot mixture; collect the gases from near the the tip of the flame through a metal tube.
The flame there has next to zero oxygen, oodles of CO2, some water vapor and of course is 80% nitrogen.
You could dry the gas mixture over fresh lye crystals or roasted Epsom salts, not necessary.
Some CO2 laser mixes even use trace amounts of O2 and/or H2O deliberately in the mix.
Dry Ice. Will freeze water vapor so it drios/filters. CostCo gives free nitrogen. CO2 lasers, yeah, pure heat. Not visible. (Esp after you burn out.your retina.)
He is going to be real lucky if he is going to get anything out of that setup. The mirror alignment setup is way to coarse and I dont think he will get a low enough vacuum with that pump setup. The light he got our of the tube was from the plasma, not laser light. This laser emits far outside of human vision.
Clearly the video was not captured using human vision :-)
No, of course not, but that does not make much difference here. Silicon based camera sensors can see near IR, up to about 1100nm. CO2 emits at 10600nm. That’s nearly one order of magnitude longer.
Only a thermal imager can see this wavelength.
Yeah, and specifically uncooled detectors, the cooled ones are usually in the MWIR range and not LWIR
And the builder admitted he may have been jumping the gun on getting emission.
If you’re serious about this: http://www.repairfaq.org/sam/lasercc2.htm#cc2toc
Oh, and
http://www.repairfaq.org/sam/laserco2.htm#co2toc
and
http://www.repairfaq.org/sam/lasercon.htm#contoc
“there’s no mention of what became of the mercury vapor or liquid therein”
Simple fix: it should be done outdoors or in an actively ventilated area.
Likely the same thing happened to that tube as any broken florescent tube, the florescent tube police come and collect the mercury and mercury vapor released!?!
The glass is very thing for use in other applications, I tried florescent tubes for a water solar heater at one time… they are fragile. I see he had the same issue and switched everything up to the purchased tube…”so i remade everything” classic!!!
I did this 20 years ago.
https://www.kontraptionist.com/post/7588645327/diy-cnc-cutting-laser-home-brewed-years-ago-in
The repairfaq was really helpful. Happy to share details.
I built one in the early 90’s for a high school project, never got it to work though. Did not have a good enough vacuum pump, this was before the advent of ebay and the explosion of relatively cheap vacuum hardware. I still have the tube.
Dude, doesn’t party balloon helium have a couple percent O2 and whatever else was in the room? Isn’t it often the flushing and release helium from servicing hospital MRI’s and lab NMR’s and stuff like that and from filling commercial tanks of high grade helium?
The way to get it right is plenty simple. Make a little chart of or simple program for target pressure in a tank as you add each gas. PV=nRT. Obviously it doesn’t need to be higher pressure than that balloon.
Oh, BTW, helium passes through the walls of those latex balloons really well. The helium content is changing fast after you fill it. It is probably half gone in 12 hours.
That little steel tank is perfect for making your mixture and storing it, in which case V is a constant so P=(n/V)RT with P in Pascals V in cubic meters, T in kelvins, R is the gas constant and n is the amount of gas in moles. You can wrap up V, R, and some constant to make a single constant for using whatever system of units is convenient. The target pressure is pressure after the gas cools to ambient temperature that you use in the calculation. See this https://en.wikipedia.org/wiki/Ideal_gas_law