[Advanced Tinkering] needed a source of fresh ozone for some future chemistry related projects, and since buying an off-the-shelf unit would be, well, just plain boring, it was obvious what to do (Video, embedded below).

The concept of the corona-discharge ozone generator is pretty straightforward — a high-voltage AC potential is presented over a large surface area, such that any O2 in the vicinity has the chance to get a decent dose of electrons ripping it apart and enabling the formation of the desired O3.
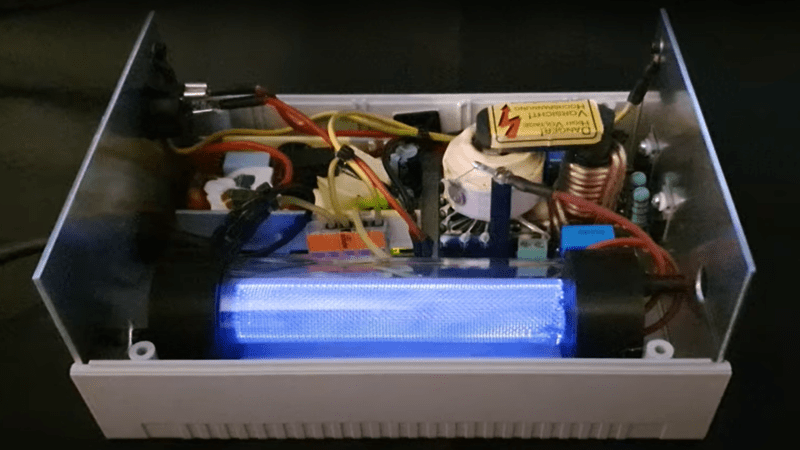
The construction is quite simple, just a pair of cylindrical metal wire mesh electrodes, separated by a glass tube, with a second glass tube surrounding the whole assembly. The use of high voltage AC allows the discharge to form by capacitive coupling across the central tube, giving a very simple construction. A pair of 3D-printed PLA end caps complete the reaction vessel, although it is noted in the video that the PLA is not terribly resistant to the corrosive effects of ozone, and time will tell whether these go the whole mileage.
Feed oxygen from an external generator is pumped into one end cap, at the bottom, with ozone-enriched gas passing out the other end, at the top, giving the gas a more complex path through the assembly and maximizing the contact with discharge. It will be interesting to see what the produced ozone will be used for in these future projects.
We’ve not seen a vast number of ozone hacks, but we’re no strangers to high voltage applications, like this interesting hand disinfection device, and this simple hack that generates a six-figure voltage with little more than some glasses of water, well not much more anyway.















Nothing like poisoning the air that you breath. https://www.lung.org/clean-air/outdoors/what-makes-air-unhealthy/ozone
better stop driving your car then
Really my guy? There are legitimate uses for O3, like killing mold, order causing bacteria, and infectious diseases. The issue is not understanding that breathing in while active ozone is being dissipated is bad. You do it in an isolated area, like but fumigation.
Yes, ozone is a toxic gas and “air cleaners” produced in the 20th century poison the air in homes.
https://www.epa.gov/indoor-air-quality-iaq/ozone-generators-are-sold-air-cleaners
Note the original post has the purpose of the device is to make ozone for experiments, not wacko health benefits
One of the first things the hacker in the video says is “ozone is a toxic gas and it should not be produced in enclosed areas”
Ozone is used to remove smells in side of car interiors. There’s much worse things you could expose yourself to than this.
Nothing like publicly demonstrating ones knowledge on a subject stops at scaremongering. Which, in case you didn’t know, depending on your company, can be much more dangerous to your health…… Unfortunately, uneducated wankers that get their info from biased shockblogs (which exist only to keep yobs preoccupied and anxiety ridden over things that have NOT been killing us for decades by cherry picking quotes and skewing graphs, yet for some reason are seen as trustworthy) then redistribute said vitriol assuming everyone else is as clueless as they are (which is incredibly offensive, IMHO) and need to be informed of the potential death around them so they can be barely functionally anxious too. Oh how kind of them….. I would say “tiktok not falling for pop-culture fearmongering challenge” but we all know people like cannot help yourselves. You NEED to find some way of raining on someone’s day to feel superior in the (incorrectly reasoned) death creeping around every corner. Stop it. Get some help. If yall think we’re judging you for how you eat, just imagine what we think of you when yall spread misinformation like this….. I mean, just because we recognize zealots like you cannot be reasoned with does not mean we are not quietly discussing how sad being so secure in your public display of ignorance you are…..
Wouldn’t it work better without the outer glass?
But then the ozone would then be able to oxidise any organic material inside the box, like the varnish around the inductors and transformers and any plastic inside the box. And also accelerate the slow oxidation of any metal inside the box. And if you put the coil outside the box without the second layer of glass then that is just asking to receive shocks. I’m guessing there is enough resistance to keep it from killing, there usually is, (but not watched the video yet).
Just watched the video, there is also the issue of arcing from the HV metal mesh to the rest of the circuit, so more insulation is never a bad idea. There could even be an unwanted coronal discharge between the metal mesh and the rest of the circuit, helping to accelerate it’s eventual failure.
I think you are being a bit too kind. The design looks like someone has studied a double dielectric discharge ozone generator and not understood it. That said, it will produce some ozone, which is toxic and nasty. Maybe it’s a blessing this hasn’t been done well.
Hello,
thank you for your critique. Like I said in the video, I am not an electrician, but I am always keen to learn. Why do you think, the design is bad? One thing I realized was, that I could have also used the inner tube, to get more surface area.
As soon as I get the potassium iodide, I can determine how much ozone the generator produces.
Kind regards
Ozone is like chlorine. Too much is indeed bad. But some is A-Ok. And in the case of chlorine (and sodium which reacts violently with water, and potassium which reacts violently with water, and calcium which reacts with water producing hydrogen gas (AN EXPLOSIVE USED IN ROCKET FUEL!!! EVERYONE PANIC!!!)) the nervous system and muscles in our body (which is mostly water) would not function.
So maybe there is a bit more to trace O3 in the surrounding air than just rattling off some line of an MSDS would have you assume. And maybe spreading fear about things one does not understand doesnt help anyone, and can even be …. gasp …. harmful 😲
Ozone , biological effect / oxidation, half life is highly misunderstood by the public at large. Ozone itself tends to stay neutrally boyant in air, and has a half life in still air of 20 minutes. It is extremely effective at eliminating mold spores and toxins in the air due to the chemical oxidation. Under heat, UV or high air flow, the half-life decreases. Much of the world uses ozone for water purification, laundry, air purification in large areas such as airports and other areas where oxidation effect is required. in fact, it has been shown highly effective at extending shelf life of fruits due to ethylene elimination/oxidation. A similar effect with high field areas and high local humidity can generate hydrogen peroxide as well.
Point me to info!
Hello,
I wanted to use pure oxygen in the ozone generator, and I wanted to be able to direct the ozone into a reaction vessel. So I needed the outer Tube to contain the ozone that is produced.
Many years ago when I lived in France, my apartment had an ozone generator mounted in the kitchen ceiling. It was supposed to destroy cooking odors. All the apartments in the building had one of these. I don’t know how common they were across Europe, but I have never seen one in the US.
I suppose it depends on what you are cooking, but I find cooking odors preferable to the smell of ozone – especially in France.
Please don’t mess with ozone, it will strip your lung lining quite easily[0].
[0] https://www.epa.gov/indoor-air-quality-iaq/ozone-generators-are-sold-air-cleaners
Not sure what you’re being brainwashed with from that page.
“In one study (Shaughnessy and Oatman, 1991), a large ozone generator recommended by the manufacturer for spaces “up to 3,000 square feet,” was placed in a 350 square foot room and run at a high setting. The ozone in the room quickly reached concentrations that were exceptionally high–0.50 to 0.80 ppm which is 5-10 times higher than public health limits ”
Shock horror, using something in a way it’s not intended may be dangerous?!?!
Next they’ll be publishing a report saying that (ICE) cars are dangerous as when used in an enclosed space with no ventilation they will suffocate anybody present.
Tell you what, don’t go outside, lock and seal all doors and windows. That’ll keep the nasty ozone from stripping your lungs.
High asthma rates immediately surround all seven of Manhattan’s bus depots among the people that live there. Why do you imagine that is?
Ground level ozone is a component of smog and a health hazard. Chronic exposure is associated with multiple adverse health outcomes.
That being said, tinkering with something like this in your garage is probably much safer than using gas powered oven/range in the home. That has been associated with 30-40% increase in child asthma rates over homes with an electric stove.
Bus depots also have another common factor, diesel exhaust, DEP’s are well documented across many highly respected medical publications as being contributing factors in lung diseases such as asthma, of course so is ozone, however, at locations where those particles would be as prevalent as they are at bus depots full of exhaust fumes all day long, this must be factored in.
Made me wonder how bad it is for your health to live in a moldy apartment? People use ozone to get rid of bad smell in a cars (usually air-con smell) and mold in their bathrooms. If you use it inappropriately, then it is a hazard. Just like fire or anything else that could be useful in one way and kill you the other way. If you want to use it for great purpose, then everyone should know pros and cons. I won’t bring my petrol/gas bottle into my house .. Smells bad. Haha.
If you feed something like this with plain old air, does it produce nitrogen oxides?
I would think so. Even if nitrous oxides are not formed in the plasma, the O3 will react with N2 in the air.
The primary product with air is still a low percentage of ozone. Ozone reacts with nitrogen oxide and dioxide to ultimately produce nitrogen pentoxide. Ozone does not oxidise nitrogen.
exactly!
This technique is from an old hobbies magazine I have. It seems a pretty klunky way to do it, no idea if it really works:
http://www.surfacezero.com/g503/uploads/612/Nitric_acid_from_the_air_Australian_HOBBIES_ILLUSTRATED_magazine_July_1950.png
When compared to the weaker double bonds in Oxygen molecules, about double that energy will break the extremely strong triple bond in Nitrogen molecules (The reason why Nitrogen in the atmosphere is mostly chemical inert. That we do not have nitric acid rain, but small amounts are produced by lightening with the natural amount of water vapour, typically 0 to 3% – but usually not enough to worry about).
498.34 kJ/mol bond energy Dioxygen
945.41kJ/mol bond energy Dinitrogen
Have a look at bigclive s site for some DIY ozone making devices.
http://bigclive.com/ozone.htm
PLA is a sucky material. If anything in that box gets warm, the PLA is going to melt. I don’t know if any other plastic would hold up better to ozone, but almost anything is better when exposed to heat.
I’ll never understand why people print anything using PLA.
Over 200C? If things in the box get that hot, you have other problems. 200C. Not even 200F, 200C.
Funny thing, PLA and ABS print at very close to the same temperature. Most injection molded plastic project boxes are made of ABS. So not that much difference.
Big difference, look up the glass transition temp for both. Abs is tolerated in 3d printing because of its superior resistances even though it sucks to print.
PLA will start to deform around 55-60C. I had a delta 3D printer built from a kit that came with PLA parts, and it warped and became useless after sitting in my hot car for a couple hours. I’ve never used PLA for anything after that.
Wow, that’s awful.
I print with PLA+, people are using it for things that get rather hot.
Man, the negativity here.
#1 – It’s for science, not to breathe.
#2 – it’s a hobby project. If you have constructive feedback, fine.
#3 – Yup, ABS is stronger… But it also gives off styrenes so hey, that’s possibly an issue for them.
Being nice must hurt for some of these people. Haha
I wonder how much Ozone this produces. The idea of using ozone to make water drinkable has been in my head for a while. Especially since all that would be further required (next to removing debris from the water, obviously) is some sort of power. Solar maybe. It would make for an interesting portable drinking water generator.
D-d-d-drink o-o-o-zone?!?! I’m getting right on the phone to some hotline or another bc you’re clearly a danger to yourself!!!! /s Just echoing the rabid safety zealot sentiment this comment section is overly saturated with, stifling curious discussion now that that overriding tone has permeated its festering thoroughly…. just like most any high-traffic discussion venue on the ‘net. Boring-ass people spreading boring-ass ideas.
[Assuming all correct safety precautions are being taken] what effect would ozone(03) have on the combustion properties of a torch, if it was fed into the system in place of/in addition to the air/oxygen(02)? [On a welding torch, through the oxygen line or on a hand torch, into the venturi] It would in theory- burn hotter? And/or quicker? Right? At the cost of degradation of the torch internals obviously.
I am not going to try this- I’ve just always wondered what effects ozone has on combustion in comparison to combustion in air/with o2.