You’ve probably had a company not support one of your devices as long as you’d like, whether it was a smart speaker or a phone, but what happens if you have a medical implant that is no longer supported? [Liam Drew] did a deep dive on what the failure of several neurotechnology startups means for the patients using their devices.
Recent advances in electronics and neurology have led to new treatments for neurological problems with implantable devices like the Autonomic Technologies (ATI) implant for managing cluster headaches. Now that the company has gone out of business, users are left on their own trying to hack the device to increase its lifespan or turning back to pharmaceuticals that don’t do the job as well as tapping directly into the nervous system. Since removing defunct implants is expensive (up to $40k!) and includes the usual list of risks for surgery, many patients have opted to keep their nonfunctional implants.
The failure of so many implant startups does raise the question of how we can better facilitate advances in implant technology while making sure there’s a safety net of spare parts and documentation should these startups fail. The Institute for Functional Restoration out of Case Western Reserve University is dedicated to designing Open Source implants for patients, and there is growing interest in standardization of some neurotechnology implants as has already happened in the pacemaker industry.
We’ve previously covered the failure of Second Sight implant company, how more general IoT companies try to get out of long-term support, and what happens when those more mundane hardware companies go out of business.













IMO, if a device is implanted in a human, there should be a guarantee that either:
(1) the device is supported by its manufacturer for the lifetime of the patient…
or (2) the full and complete design/support documentation for the device is released for others to support.
I can sort-of live with some proprietary widget like a home automation system suddenly ceasing to work. It’s a nuisance, but it in most probability will not kill me.
If that device is implanted, side-effects of the manufacturer’s cessation of support could vary from nothing, minor annoyance through to death. Maybe it’s fine without manufacturer support for a little while, but then develops a fault, how does it get fixed?
Is it possible to remove it? In some cases maybe not — so the end user must be able to find someone who can help them fix it in the situation the device malfunctions. Ideally, that should be the manufacturer, but if they’re not able/willing to help, then the end user needs to find somebody else — ideally someone with medical implant experience — and that person will need the documentation of how the device works. Get it wrong, and the patient could suffer terribly, possibly fatally — there should be NO guesswork.
The only way to ensure this, is to ensure full documentation is accessible, or to mandate the OEM continues support until the patient dies.
Mandate it but when they go bankrupt, how do you enforce that mandate?
You require the source to be held in escrow in the event the company goes under. This is already a thing in the software world.
One might even go further, and demand that the complete documentation is available for every medical device, before it is accepted for use. The manufacturer can protect their rights well enough with patents.
Or the FDA could hold the documentation in escrow if it contains trade secrets, with a legal requirement to disclose it if the company drops support. That way the docs don’t get lost in the shuffle if a failing company.
In fact most documentation, code, support is already well known as most medical certification requires it.
But nobody want to support a dead product, because nobody want to pay for it…
Could be a law that if support cease, opening up al the documentation including source code and schematic would be mandatory.
I wouldn’t thin that cyberpunk-like biohacking comes that fast. it’s already there, at least for people with implants and those who help them maintain them.
This thread, as it is while I’m writing this, is maybe the most hope-inspiring comment-thread I’ve ever seen on hackaday. Imagine so many folk actually thinking about others’ wellbeing.
Escrow of documentation/sourcecode within/by the FDA makes a lot of sense, for all FDA-approved devices, medications, etc. And maybe a second-party’s assembly of the device, or synthesis of the medication, from that documentation alone, to verify its completeness, before FDA approval.
Similar could apply elsewhere, FAA, UL…
This could lead to great things. Here’s hoping.
This is why nobody should be even thinking about using Neuralink unless and until laws are passed to ensure that implant tech cannot become abandonware
That seems a little harsh. It’s not like we have seen other companies managed by Elon Musk behaving in capricious ways where they suddenly behave erratically, such as firing large swaths of employees, unilaterally backing out of contractual obligations, and deserting users without cause.
Your sentence could lose a lot of weight:
“Nobody should be even thinking about using Neuralink”
Frankly, it’s you that needs to lose some weight. I know your hell, let’s not introduce it to everyone randomly.
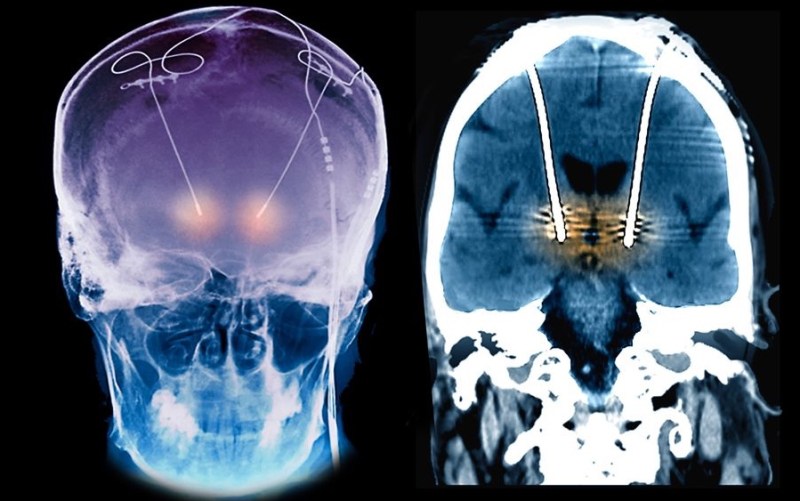
Having placed many hundreds of implants in people, I have some experience in this area. It is almost never necessary to remove the electrodes, whether they be in the brain or near the spinal cord – though they can be removed usually easily. The brain and the spinal cord form a scar tissue (gliosis) around the electrodes and they generally slip out quite easily. It might take hours to get them in the right place to start with, but removal is often quite quick.
The “pulse generator” is usually the main component of these systems, and is usually placed in the anterior chest wall, like a pace maker. These are very simple devices, for the most part, and are almost trivial to access and replace as needed. Many companies have adapters for other companies wires, but I do agree that common standards would be helpful.
As devices, hopefully, become more advanced, and more responsive I agree that support will be needed. Currently, most devices deliver a standard frequency of pulses, some have timers, but are relatively simple.
The images used are for treatment of tremor, not for the ATI treatment of headaches, which I have never done, and it might be more difficult for the removal of the electrode around the superior ganglion (peripheral nerves scar down differently and can be a pain to remove), but, it is rarely needed to remove an electrode unless there is an infection.
That’s good to know. My father has Parkinsons and we have been investigating Deep Brain Stimulation for his condition. As scary as surgery is, the fear of losing support for an implant that is affecting your brain is quite scary. Even the software support for the programing device at a Neurologists practice could eventually be cut or be incompatable with future operating systems. Even the thought of having an MRI after getting the implant is a big concern.
Same as the environmental disaster of medical equipment with radiation sources being abandoned. In the Goiânia accident, a scanning machine with such a source was abandoned in a doctor’s office. When it was scrapped, there was no one to warn that the box of blue powder behind thick lead walls would lead to a whole neighborhood being irradiated.
When an expensive, mostly bespoke medical device requires professionals to handle it safely, it must have a cradle-to-grave plan with organization separate from the company that produced it, both to limit risk and to ensure proper liability.
Goiânia accident was caused by theft…the original device had plenty treefoil signs on and in it.
To all those saying “nah, there should be a law against this”: look at the insulin medication prices in the USA and at the efforts big pharma does to protect their margins (look up big pharma and Synema to see what I mean).
Laws can sometimes be bought, alas.
The high prices of pharma are at least partially because they have to internally insure themselves against coughing up to sort out stuff like this should one of their own devices stop working. And due to the work involved in making sure they’re as least likely as possible to end up liable for something. So big pharma may be expensive, but is at least pretty reliable overall. Obviously the litigation culture in the US drives up your prices compared to other parts of the world.
But this means high prices and very slow time to market. Which isn’t good for patients.
In the UK we have the FCA guarantee to protect people’s money in the banks. Something similar for implantable tech might make sense here. An independent guarantee that issues with any certified tech will be resolved (yes, that might mean removal rather than continuing support, but better than nothing).
As the recipient of expensive and cutting edge drug for a non-life threatening condition, I am actualy thankful for for-profit medicine. This drug wouldn’t even exist without a companies ability to profit, and patents expire, so there will be cheaper generics in the future.
That said companies invloved in implantables should have a bond and schematic/source escrow to pay for removal or modification in the event the parent company fails.
Are you sure about that?
Salk wasn’t looking for profit – & nor were Fleming or Jenner.
This world needs less naked avarice & a little more brotherhood-of-man…
I have a cochlear implant and fortunately the company has been around long enough that I think it’ll be around for a while. The internal implant can last decades. If my company shuts down, hopefully insurance is still available to switch to another company and replace the implant. If not, I’ll resort to ASL and hopefully I’m retired by then since verbal communication is critical to my job. Good article!
The problem is that the act of installing an implant causes damage, so you don’t want them replaced if you can help it. Much better if there is a way to ensure continued use of the implant after a company goes out of business.
1. There should be a mandatory filing of all technical documentation with regulators.
2. Bankruptcy laws should be changed to prioritise transfer of technology to another provider in proceedings above paying creditors. Particularly if another company buys intellectual property, a liability to support existing patients should be included.
3. If the intellectual property is not sold, all technical information should be placed in the public domain.
You mean a pack of 20 menthol cools isn’t enough ?
https://www.youtube.com/watch?v=1c7FYxTa2mQ
Consumers of these devices should refuse to buy them unless the seller puts the full documentation in escrow to be released if and when support is dropped.
This would do a lot to align the interests of the companies with those of the consumers.
Difficult to organize, though.
I wonder how many consumers it would take to force the manufacturers’ hands.
If only people getting implants could choose to not get them because they might be abandoned later! I bet that there’s a section in the paperwork for ‘end of product life’. If you legislate a bond/escrow requirement then you will simply be placing a stumbling block in the way of development. If you sign up to be a science project, a medical experiment even, you already KNOW there are many risks before you ever start.
It does seem rational to me to say ‘hey, this thing you’re putting in me, can I get a schematic and stuff for it before it is put in?’ although they’ll probably say ‘no, we don’t want to’ or ‘we’ll experiment on someone else’. Do you get a data sheet like what you get for your drugs from the pharmacy when you get an implant?
As a recent recipient of a neural implant, the timing of this article and the questions it raises are succinct. I feel the “escrow” solution is equitable and reasonable.
“Now that the company has gone out of business, users are left on their own trying to hack the device to increase its lifespan … … Since removing defunct implants is expensive (up to $40k!)”
Call me crazy, but “don’t get experimental implants from a startup unless you know you can get rid of it without pulling the thing out of our head yourself” seems to be common sense type thinking.
Yes, I am very aware that some people are despearate for relief and are willing to try anything because it’s their last hope. But this is one of the things governments exist for, right? Regulation can make it so before anyone is allowed to be used as a guiny pig by a startup, the startup has to put away enough money into a fund that will be utilized to pay for the medical procedures required to get these things out if the place goes belly up and other similar situations. It could be setup that once these things become main stream and eventually covered by insurance the company can take those funds out and use them for something else.
I am not talking about the government controlling what you can and cannot allow to be done to your own body. Only that in order for it to be done, there needs to be a guaranteed way, at least financially, for you to get it undone.
You might argue that this would limit the development of new tech that could help these people. And you’d be right. Now take another look at the iamges at the top of this page and think about it. Do we let more or less people suffer until it catches on to the point it becomes something main stream enough that is covered by insurance? Because I don’t think the size of the medical bills involved in having these things removed and possibly treating what was made worse will ever get any smaller.
In the future, it’s sad to say, a voice will be heard in a person’s head saying.. ” the warranty of your neural link is about to expire… “
If the company won’t release the full documentation then the people with this device implanted should pay a visit to its offices before nothing remains and seize for themselves any information which they could then provide to volunteers who’d be able to use it for reverse engineering the implants. Until laws are passed to put such tech documentation in to independent third party escrow services patients will have to enforce righteousnes for themselves.
Also, what proportion of the device is actually implanted, all the “smart” parts, or are those outside the body in a casing while the in-body elements are the “sensors” and “actuators” only. If the “smarts” are external could reverse engineering be done on a working one so as to copy the protocols needed to understand the sensors and command the actuators, and thereby build an open source external replacement smart section?
I’ve been looking into these and a lot will have a cbox implanted in your chest like a pacemaker. All programming and adjustments to the device is made with another box outside the body, usually connected to a computer. The external box is held over the internal one during programming or reading. There would be some FCC documentation available and deduction fn communication methods via some type of NFC, maybe via SDR. But without proper documentation, not knowing the proper commands and variables could result in nothing at best or a fatality at worst. Reverse engineering a device like this is fraught with danger
No refunds