Hydrogen is a useful gas. Whether you want to float an airship, fuel a truck, or heat an industrial process, hydrogen can do the job. However, producing it is currently a fraught issue. While it can be produced cleanly using renewable energy, it’s often much cheaper to split it out of hydrocarbon fuels using processes that generate significant pollution.
There are methods to generate hydrogen more efficiently, though, in a clean and sustainable process. that also produces useful heat and oxygen as byproducts. The key to the process? Concentrated sunshine.
Solar Split
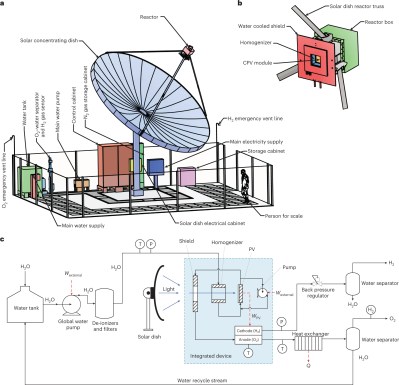
Hydrogen touted as a clean fuel of the future, by virtue of the fact it can be burned or used to make electricity with minimal to no emissions. It’s touted as a potential fuel for cars, trucks, trains, planes, and even construction equipment. However, while the hydrogen itself is clean, generating it often isn’t. The race is on to find a clean method of producing hydrogen at scale, with researchers investigating everything from nanoparticles to advanced pyrolytic processes. Whenever you hear people talking about “green hydrogen,” this is what they mean: hydrogen produced without any nasty greenhouse emissions.
With an eye to producing exquisitely clean hydrogen. researchers have demonstrated a pilot plant on the kilowatt-scale using solar hydrolysis technology, as per a paper published in Nature. The system runs on municipal tap water, which is run through multiple particulate filters and deionizers to prepare it for the reactor. Inside the reactor, the deionized water is heated by light captured by a 7-meter diameter parabolic mirrored dish, which acts as a concentrator to maximise the solar energy that reaches the reactor. This light not only heats the water, but also reaches a photovoltaic panel which provides energy to run the PEM electrolysis cell, which is what actually splits the water into hydrogen and oxygen.
The key to the system is the double-purposing of the solar energy input. The most basic idea is to simply use solar energy from a photovoltaic system to power a PEM electrolysis cell. In this case, though, the solar energy is also used to heat the water which drastically improves the performance of the electrochemical process.
A holistic approach also maximises the economic value generated by the system. Waste heat from the system is captured with a heat exchanger where it could be used for a variety of external heating purposes. Additionally, the system doesn’t just output hydrogen, but oxygen as well. While this isn’t directly useful as a fuel, it’s still useful for a wide variety of industrial and medical applications.
The pilot plant produces roughly half a kilogram of hydrogen per day. That’s enough to power a single hydrogen car for a European racking up fairly average annual mileage. Alternatively, such an installation could provide roughly half the electrical demand and over half the annual heat demand of an average Swiss household. Realistically, though, straight photovoltaic solar would be far simpler in this instance.

Plans are already in place to build a larger system at the multi-hundred-kilowatt scale, which will produce hydrogen for use in a Swiss metal production plant. It will also supply oxygen for medical use and deliver hot water for use at the factory.
Incidentally, if you’re interested in designing your own similar system, help is at hand. The École Polytechnique Fédérale de Lausanne (EPFL) has released the Solar PhotoElectroChemical Device Optimization tool, or SPECDO for short. Essentially, it’s a web page full of calculators that determine the performance parameters of a given solar hydrogen generator. You’ll need to be pretty nifty with your engineering though, and find a way to source an effective PEM electrolyser for your design.
If hydrogen does become a mainstream fuel of the future, solar photochemical processes to make it efficiently will be key. There’s no point in spending huge sums of money to convert transport and industry over to hydrogen fuel if we produce it in a way that still creates greenhouse gas emissions, after all. At the same time, this research shows that hydrogen still isn’t a silver-bullet solution to all our problems. It requires significant engineering and finesse to come out cleaner than the fuels it’s intended to replace.
















Energy is stored as hydrogen. What specifically are your concerns regarding lifespan ?
If I read it correctly, TG implies that photovoltaic Solar has longevity and storage issues, not hydrogen.
Elemental hydrogen has storage issues as well. In gaseous form it’s very bulky, high pressure, and hard to keep contained. Liquefying it increases density but adds its own set of issues.
Unless… you combine it with carbon (eg. liquid propane) or nitrogen (eg anhydrous ammonia). Those forms have pressures and densities such that seasonal energy storage (production in summer for use in winter) just might become viable.
Yes we prefer out hydrogen stored using carbon chains such as C12H23 as it is far safer to store, transport and use.
There’s a plant south of Lincoln, NE run by a company called Monolith. It’s intake is natural gas and it’s output is hydrogen and carbon black.
Why bother? Well, for one carbon black is produced overseas by smothering a hydrocarbon fire – that method is an environmental disaster done deliberately. As it’s done by Monolith it produces no carbon dioxide and originally they’d planned on using the hydrogen to power turbines to produce electricity to power the process. In practice they are using the hydrogen to make anhydrous ammonia. In the future when there’s more of a market for hydrogen I imagine they could switch back to having hydrogen as one of the outputs.
Well, the hydrogen will be mostly used to produce ammonia anyways.
“ Yes we prefer out hydrogen stored using carbon chains such as C12H23 as it is far safer to store, transport and use.”
Well, when you can do that affordably without pulling the C out from a hole in the ground you’ll be famous. Maybe even win a Nobel. Until then – like most of us – you’re just part of the problem.
Simple. Just store it as a solid. That can’t be hard right? Just get it cold enough with enough pressure… hmm
There have been recurring efforts to use pure thermal cracking of water into oxygen and hydrogen and some have been fairly successful. There’s been one running in Brazil for almost 20 years. It runs into materials limitations since it has to run at about 3000C, but the rest of the system is pretty simple.
If we can’t make fast enough battery progress, hydrogen motor/generator sets as range extenders might be viable, particularly for stuff like large trucks. Maybe trains, too, for where overhead catenary isn’t viable.
When you have hydrogen you are more then half way to CH4. There have been many trials of using just electricity, air, and water to create CH4. Hell.. European HELMETH generated sufficiently pure and dry CH4 with pressure sufficient to be directly injected into a standard gas network.
You know what we have in Europe and also elsewhere for CH4 which we don’t for stupid H2?
– Already built massive CH4 transportation networks.
– Already built storage so f’ing large that it can power whole f’ing continent for not minutes, not a few hours during night, but for a whole f’ing days!
Batteries and Hydrogen be damned.
And also produce a f’ing amount of CO2 when burnt, and if not burnt well, while being released in atmosphere, work 40x better than CO2 to heat us well.
You absolutely don’t understand basic chemistry… like at all.
Absolutely exactly like f’ing physically exactly the same amount of CO2 is released which is taken during production from air.
Where do you take electric power for the pruduction is what matters and this problem is absolutely exactly the same for hydrogen.
Actually you can blend H2 into natural gas, in fact “town gas” used to have H2 in it already.
But yes, capturing C02 from the air you’re processing will be carbon neutral, if you then burn the CH4 you generate. Or, you could make ethanol.
Yes. You can go even to much much longer hydrocarbons and create diesel or basic plastics like PP. Each step however is going to have its inefficiencies. Don’t know where lies the cost-benefit middleground but ethanol sounds nicer than CH4 for storage.
sounds better than solar panels
“Sounds”, but if you look at the efficiencies, charging EVs is gonna be a lot better (i.e., cheaper) than gassing up with hydrogen. Which has to be compressed, and transported, and put into a fuel cell, or worse, an ICE.
Depends on the EV. It’s not that much more efficient, especially if the vehicle is on low duty (big battery, city car) because of the huge embedded energy cost of batteries.
A factor of 3 is not a small difference. 50 kWh will get a Mirai 66 miles, a comparable BEV just shy of 200.
The cost for the raw materials (carbon fiber, resin by weight) to make just the storage tank is comparable to the price of 60 kWh of battery cells. But the FCV still has to buy the fuel cell itself, plus some amount of batteries to handle peak loads.
And chargers are a whole lot cheaper to make than a hydrogen dispenser.
Both systems are pretty useless close to half of a year in the polar regions, If one was to take advantage of the summertime in polar/subpolar regions, this method of producing H2 certainly would be the logistically easier way to provide propulsion for vehicles during the dark cold winter.
I wonder if they would get better thermal efficiency using enclosed parabolic troughs instead of a single dish. It would certainly lower maintenance costs where the mirrors and dust are concerned. There would also be a little boost from the enclosures acting like green houses.
The solar towers that use a sodium potassium mixture as working fluids could be converted to cracking water instead of producing steam for turbines. Though it’s been a decade since I flunked thermodynamics so I have no clue what that kind of system would look like let alone what its efficiency would be.
They need a 1000 times concentrator to get the water to split. Also, a cloudless day.
In a hybrid system with steam electrolysis, they mostly just need it to boil water.
A big issue with electrolytic cells using liquid water is the purity – the membranes get stuffed with junk, so a lot of energy needs to go into cleaning the water first. The steam plant works as a distillation device, and while the steam is hot it requires less energy to split. The amount of heat you put in is reduced from the electrical input. The only need then is to de-ionize the water to prevent scale buildup in the steam generator.
However, the hydrogen comes out at a relatively low pressure and needs to be compressed. In liquid systems, you can more easily compress the water to a very high pressure so when it splits, the hydrogen comes out at tank pressure, so it’s a give and take.
“float an airship”? Yeah, that’s proven technology.
Well, it -did- work…. for a while.
It was safer than airplanes.
I find it amusing that SOMEONE ALWAYS cries hindenberg at the mere mention of hydrogen balloooning. The first manned hydrogen balloon flight was December 1, 1783. First death (2 deaths actually) June 15th 1785 in a Roziere Balloon, a hot air/hydrogen dual envelope hybrid….it crashed but didnt explode. The Hindenberg (1937) killed 26 immediately with 10 more succubing to their injuries in the days and weeks that followed. 62 passengers/crewmembers survived 37% fatality. The worst dirigible explosion of record happened 4 years earlier when 73 of 76 members of the USS Akron perished. 73 the worst death toll despite military use of manned hydrogen balloons from Napoleon to World War I. But the hindenberg pretty much ended 154 years of hydrogen balloon advancement.
The first powered heavier than air flight happened dec 17 1903. The first death September 17, 1908. Japan Airlines Flight 123, the most deadly single plane crash killed 520 with only 4 survivors in 1985. We still use the 747. Without digging too deep in detail.. This list despite its label includes 101 incidents, http://www.planecrashinfo.com/worst100.htm, The 100 worst aviation disasters, excluding the Towers, claimed 19659 lives….and thats just the worst of the worst, the oldest of those 1962. We still fly planes.
Im with Walternate….nothing wrong with a little h2.
There’s a difference with mechanical failures, design flaws or human errors, which can be fixed and controlled for. We don’t ban cars because someone crashed one into a ditch – we can make safer cars that won’t kill people in a crash, or install crash barriers, or lower the speed limits, or require better driver training, or…
With hydrogen, there is inherent danger that we can’t really do anything about. It’s just down to luck when the next Hindenburg would go down in flames.
keep blowing that horn. Enjoy your Bliss!
And there is inherent danger in Petrol and all the vapours it puts outs so readily, and ammonia, and….
We use stuff that can be dangerous all the time, there is nothing unique about hydrogen that makes its ‘we can’t really do anything’, we just have to do different things when dealing with it.
Hydrogen as a fuel and for its lifting properties has been used for a very very long time anyway, and mostly very safely with a few dramatic but rather over hyped accidents. Much the same as nuclear power really – yes in the past and with old designs there are risks and those risks can lead to rather dangerous problems. But the same is true of everything else look back at the early versions and they are relative death traps and now can be operated safely.
The old airships vented hydrogen like crazy near various sources of ignition and like all vehicles from that time, they didn’t have modern safety standards in mind. Despite that, when they did sink they often did it so slowly that a lot of people survived. Imagine getting on a jet airplane today and finding out there were no oxygen masks, no rules about keeping exits accessible, no safety procedures, no redundant systems, etc. You’d wonder if you were about to die because someone forgot their wrench in the engine while they were halfway through tightening some bolts. Or it’s only a matter of time until you hit birds/hail/turbulence and go missing, presumed dead.
I could imagine a modern airship might do a number of things to protect passengers in the event of a failure. Detaching the cabin and using parachutes and the kind of airbags that mars landers have used might be useful. Or maybe for niche uses, something might be electric with enough thrust and battery power to slow its descent using nothing but the fans. I’m assuming we would never intentionally vent the lifting gas nowadays – helium is expensive and not renewable – so anything that causes the lifting gas to be exposed to atmosphere is a problem that would have affected the airship either way. As such, the only difference is what happens if it gets set on fire at that moment – maybe from a lightning strike? Maybe we would be required to keep hydrogen away from the outer skin, and do all sorts of things to protect it in the inner volume. The extra lifting capacity leaves room for extra safety measures.
What, the danger of ignition? Gasoline too is flammable. And tanks storing it have a “Flammable” plaquard attached with little graphic flames, for those who are illiterate. Add a “NO SMOKING” sign and there ya go, problem solved.
you are absolutely right
Your data in this comment is missing one important number; how many travelers/flights is that out of, or what percentage risk is there. If there were 100x the deaths from planes, but 1000x the number of passengers/flights, then, that would still make it 10x safer per flight/person.
Its still used in aerostat applications where helium is too difficult or costly to get to the ground stations. I saw a demo back in 2009 where they were firing .50 cal tracers through a decommissioned hydrogen envelope (balloon). The first dozen or so shots were extinguished when they encountered the hydrogen only environment inside the skin of the envelope. Took a while for the H2 to mix enough with the air to burn.
Everyone likes to reference the Hindenburg, and for good reason, but I think we could overcome the engineering flaws that lead to that disaster. The two best solutions I’ve seen are active gas monitoring and venting, and inert gas “shield” envelopes surrounding the hydrogen ones. Fire shielding has gotten a lot lighter too in recent years.
Lastly, hanging the gondola in an entirely separate fuselage/pod under the gas envelopes might mitigate the risk of burning to death. Equipped with those massive rocket deployed airplane rescue chutes the gondola could jettison the burning envelope and deploy the chutes once clear. Tack on some external air bags like they used for the Mars rovers and now you have redundant safety systems.
… not that I’ve been thinking about this for a while.
Since hydrogen really likes to escape any thin membrane, it’s a bit trickier than surrounding it with another gas. It will diffuse right through anyways.
There are a lot of membranes like mylar that can last months or longer without much loss (like party balloons). It wouldn’t be hard* to have a monthly maintenance that filters the shield gas and/or just drain and replace it.
I just saw your comment after posting mine – looks like we have similar ideas :D
Aye!. Lets start an airship company.
Step 1: kickerstarter campaign with a retired NASA engineer to give us some cred
Step 2: …uhh? Balloons?
Step 3: Profit, or a fraud investigations which ever comes first
Step 4: Solar-powered laser broadsides at extreme altitude.
I understand there is an energy budget here, but filtration over distillation – which produces chemically pure water? Many filtering systems require significant overpressure to function properly, such as reverse osmosis systems, at scale. I am curious regarding the energy needed for pressure filtration vs distillation.
If you take into account that takes more energy to turn 1kg of water from 100C to water vapor than to heat 1kg of water from 0C to 100C, you will see distillation will be pretty power intensive.
You need pressure? Use gravity! A tower with the water input at the top and the filter at the bottom does the filtration almost for free.
Only if you ignore the energy required to lift the water to the height needed to provide the required pressure head. There is no free lunch.
Find a location to build the plant at a lower altitude than the natural water source it taps. Pumps to raise water to a tank could be solar powered. Solar not working at night wouldn;t be a problem since the solar concentrator would also not be working at night.
But, if you want to cool the output, in the case of distilled water, you want to cool the steam/water output, which means you can transfer almost all that energy back into the source water with a heat exchanger. Then you only have to add back whatever energy was lost by inefficient processes.
They’ve been doing this process to desalinate water on an industrial scale for a long time.
This system deionizes the water in addition to plain filtration. Plain distillation carries anything volatile enough across with the water, and it turns out distilled water is still very slightly conductive unlike deionized. If you have waste heat, distillation could make sense, but for example for lab use you often find filtration systems where the later stages have ion exchange resin beads and activated carbon and such.
Distill out anything more volatile than water first. Then distill the water out from anything less volatile. Then use filters to remove whatever’s left which is too close to the same vapor point as water.
Vacuum distillation is done on an industrial scale with maple syrup processing.
In days of old, maple tree sap had its excess water all boiled out in large, shallow tanks with fire heating. Wood, charcoal, gas, whatever made a flame. The trick with hand fed fuels like wood and charcoal was keeping the temperature just right to boil out water but not burn the sap. (Laura Ingalls wrote an excellent description of the process in “Farmer Boy”.)
Now they run lines throughout a maple tree orchard and use vacuum to pull sap to the collection tanks. The sap is put into tanks where a vacuum is pulled to suck out a lot of the water. Then the sap is boiled to get the water content down to make the sap syrup.
The issue with that is they’re not getting the water content as low as was done with the old wood fired tanks. Most 100% maple syrup sold now is too thin, too much water left in it.
Who does that?
Reverse osmosis is how it is normally done industrially to make maple syrup.
Your idea of boiling out anything more volatile makes little sense once you learn about azeotropes.
Fractional distillation has downsides, and usually there’s only a little bit of volatiles in common water sources. Instead of doing two simple distillations before filtering, it’s easier to just do one, even if you could have gotten more life out of your following stages by doing the extra step.
I have family whose syrup was done the traditional way, with pails on nails and a serpentine path in a shallow tilted pan over wood in the ‘sugar shack’. Theirs has always been darker than the average commercial stuff, but it takes more effort to get it very dark – even before it’s burned it’s harder to work with, from thickening if nothing else. I’ve thought about getting the last of the water out by vacuum, since it’s cheaper than getting the bulk of it out that way and I think the heating matters.
I don’t know that I could get the same flavor even then. There’s a component of the darkness that’s from your choices in tapping and the conditions of the trees; if your trees are putting out sap with more sugar per flavor, then when you concentrate to a certain level of sugar that’s going to carry through. You might see a difference along those lines across the season. But even apart from that, getting a bit of caramelization without going too far mustn’t be as economically favorable as not bothering. There’s a big selection force for the lightest grades of syrup. The scale puts a light “A” above a dark “C” or “D” in people’s minds, so the darker stuff gets used more commercially instead of retail.
Ans so the people who prefer a darker, thicker, somewhat caramelized maple syrup can’t get the syrup they want anymore. :( They have to put up with the fake stuff that’s all made to mimic what the real stuff should be.
Hydrogen fuel cells are becoming more and more prevalent with a few companies trying to implement the product into the commercial sector – good for future emissions on a global scale if adapted.
That dish intercepts about 40 kW of solar power. Tracking the sun horizon-to-horizon yields maybe 10 hours of collection on a good summer day, or 400 kWh.
That half-kilogram of hydrogen it produces is 20 kWh of stored energy.
5% efficient.
Attaboy. Good start.
Perfect for a government funded program.
B^)
Only in some parts of the world do you get 10 hours of sun per day. Where I live that happens a handful of times per year. The rest of the time, we have some level of clouds. PV panels still work well enough, but mirror concentrators are mostly useless.
I was going to mention that, but I see that they actually blocked most of the sunlight with a reflector with a hole cut in it – so that they could test on a smaller scale than their actual dish. So the efficiency is better than it looks.
I’m usually (ok always) pessimistic but even at low efficiency, at least this aims to offset the single biggest issue with renewables: storage of energy is a kinda useful form. Even a low 5% is better than, say, 0%. And a mirror and so on don’t require mining weird metals and stuff. Yeah storage is hard but we already do it as a semi-mature technology. I love it.
So it runs on tap water…as long as you remove everything that isn’t perfect water, down to ions from it?
What a stupid statement.
Apparently I drink ocean water every day.
It just needs to evaporate, rain down somewhere, and end up in my local water system. But we’ll just gloss over those intermediate steps.
To generate 1kg of hydrogen by electrolysis, compress it to 700 bar, and chill it to standard FCV dispensing temperature takes a minimum of 50 kwh of electric power. That amount of hydrogen when fed to a Toyota Mirai will get you 66 miles (epa rating)
The same amount of electricity fed into a comparably sized BEV will take it ~190 miles on the very same EPA test. Close enough to 3 times the distance.
“But the cost of the batteries”. The hydrogen tank will cost more than the battery pack. The raw materials alone (carbon fiber, resin, by weight) NOT including the cost to manufacture the tank is comparable in cost to 60 kwh of batteries ready to install.
This is before you buy the fuel cell and batteries. (Yes, a fuel cell vehicle has some amount of batteries, just like a hybrid car. You size the fuel cell to something comparable to the average power needed, and use a small battery pack to handle peak needs.)
The controller and drive motors will be comparable in price between the two systems. The onboard AC charger of the BEV will be a lot cheaper than the fuel cell.
This^ .
Nicely summarised also in an InsideEVs article way back in 2017:
https://insideevs.com/news/332584/efficiency-compared-battery-electric-73-hydrogen-22-ice-13/#:~:text=Efficiency%20Compared%3A%20Battery%2DElectric%2073,Hydrogen%2022%25%2C%20ICE%2013%25
Unlike with battery technology, making hydrogen tanks is a function of scale, once the manufacturers started making enough of them, the tank prices would drop drastically since there are comparatively few bottlenecks in steel bottle making. In winter, after having parked the car in 40 below for a week, your EV will move a few miles, if you are lucky, before giving up the ghost. Provided you have a proper lead acid battery in your hydrogen powered vehicle, it will start without issue. Fuel cells are still immature, much easier to run an ICE on hydrogen.
“There’s no point in spending huge sums of money to convert transport and industry over to hydrogen fuel if we produce it in a way that still creates greenhouse gas emissions, after all.” Please stop treating that “food” for plants, CO2, as the source of all the evil in the world. Cars with combustion engines produce much worse pollution, i.e. nitrogen and sulfur oxides, carbon monoxide, smog, unburnt fuel leakage and noise. All that in places where people live. And it’s not so easy to filter it in cars like in non-moving installations. Of course green hydrogen is better, but electric cars with hydrogen fuel cells would solve these problems, even if it comes from natural gas.
This is cool and all, but kind of disguised ad being something different.
The main purpose of this approach should be to compare against a simpler pv+electrolysis, but that’s not mentioned.
Cost vs efficiency, robustness, emissions to implement etc.
I should perhaps clarify that i like it though, I’m just mystified how it came to be presented like this.
Also the main question about the working principle of pv+electrolysis in my mind, what’s the lifespan of the electrolysis? Will it keep up with pv’s 20+ years? Are there any consumables, and ate they expensive?
I have not seen this mentioned anywhere!
Here’s a film which shows some “retrotechtacular” versions of experimental solar hydrogen production in 1984:
https://csiropedia.csiro.au/hydrogen-power-1984/
Move over solar panels, concentrated solar reactors are here:
“Haussener and colleagues are now busy scaling up their system further in an environment where individual reactors are deployed in a modular fashion, like trees in an artificial garden… commercializing the technology, and [] working with a Switzerland-based metal production facility to build a demonstration plant on the multi-100-kilowatt scale.
Another future direction for the team could be to develop a similar system to convert CO2 into CO, ethylene or other products plus oxygen. ‘This would allow us to valorize CO2 and produce other precursors for industrial processes,’ Haussener explains. ‘For example, ethylene could be used in green plastic production, and CO together with hydrogen for liquid fuel production.’”
https://physicsworld.com/a/concentrated-solar-reactor-generates-unprecedented-amounts-of-hydrogen/