If you think of metals in a battery, you probably think of lithium, mercury, lead, nickel, and cadmium. But researchers in Australia and China want you to think about aluminum. Unlike most battery metals, aluminum is abundant and not difficult to dispose of later.
Their battery design uses water-based electrolytes and is air-stable. It is also flame retardant. The battery can provide 1.25V at a capacity of 110 mAh/g over 800 charge cycles. The idea of using aluminum in a battery isn’t new. Aluminum is potentially more efficient since each aluminum ion is equivalent to three lithium ions. The batteries, in theory, have higher energy density compared to lithium-ion, but suffer from short shelf life and, so far, practical devices aren’t that close to the theoretical limits of the technology.
Aluminum ion transport is slow, however, so batteries made with the metal tend to have low cathode efficiency. Organic polymer cathodes can help but have their own set of problems. The new battery addresses some of these concerns, but, of course, it remains to be seen if this technology will be competitive with other technologies.
There are several ways to make batteries with aluminum. Some take nothing more than thin air.

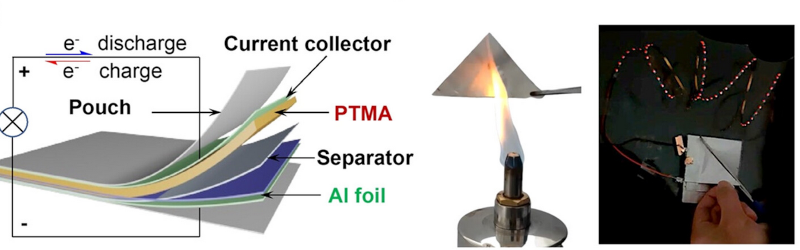















Time will tell if this battery will be practical.
Good luck getting it recycled. Even with the massive amounts of expensive metals in an old hdd, you dont get anything for it unless its disassembled. Still more trash, maybe slightly less bad trash, but future garbage none the less… be nice if designers could factor in recycling, but then again that would require stronger right to repair bills.
Shelf life and number of recharge cycles are big issues they will have overcome before even being considered.
Low price, easily recycleable, and replaceable standardized cells may also work?
A functional battery technology not based on exotic materials would be a huuge thing. I can’t help feeling hopeful every time a see news like these…
I would be happy replacing and recycling a $20 battery once a year…
Not for my watch!
B^)
Doesn’t lithium have average of 500 charge cycles before there’s noticeable degradation in capacity? Laptops and portable game systems that are used a lot and charged often will show drop in usable power time after a few years.
AFAIK only lead-acid doesn’t degrade from charge cycles but they do go bad if they were discharged too much. It’s also quite heavy and very rarely used in laptops. A well maintained lead-acid battery have lasted for many years without showing reduction in total capacity.
It depends on how high you charge it. You can certainly destroy a lithium cell in just 500 cycles but reasonable voltage limits can make it last 1,000 – 2,000 cycles.
Lead-acid batteries on the other hand are destroyed in less than 500 cycles if you discharge them more than 20% off the top. The deeper the discharge, the fewer the cycles you get out of it.
If you have an apple macbook pro they kindly put a 1,000 charge limit on your device with non-replaceable batteries (because they are glued to the keyboard) and will shut you down even at at 100 percent charge. This is for your convenience.
It depends on the cell chemistry and how well the battery temperature is managed. Standard Lithium Manganese Cobalt cells manage 1000 to 2000 full cycles before significant degradation. Lithium Iron Phosphate handles around 5000.
It literally says 800 charge cycles. I have never charged anything that many times
Wow, I’ve definitely run my phone down to ~20% and charged to ~100% at least 800 times. At least once a day for 4-5 years since I last replaced it, and I got it used.
My EV battery is the only other one I can think of, a commute every work day for 5+ years with a full (or nearly) charge and discharge every day. The EV in question has an over-engineered liquid cooling system and charge module that keeps it around 20% from either maximum charge or discharge. Still gets within 10% of the range it had new.
I have a Casio solar watch that charges every day and has done so for the last 20+ years, so ~7500 charges (roughly twice what the instructions call out for battery replacement BTW).
That’s not 7500 cycles, though, unless it was every morning and full every evening.
Recharge cycles I get. You wouldn’t consider a battery for any use if it has a short shelf life?
Why?
I think a cheaper and/or more environmentally sound battery whose only weakness is shelf life would be VERY useful… in the right scenario.
For example, how about home power storage?
If you are charging them every day by solar panel to run the house at night well… as Annie sang, “The Sun Will Come Out Tomorrow”. How much shelf life do you need?
Even skipping the panels, in some places peak and off-peak time electricity is priced differently enough that even with the inefficiencies involved one can actually save money by charging from the grid during off-peak hours and running from battery during peak times. Again, it’s a daily cycle with maybe a difference in weekday/weekend schedule.
In such uses who cares if the battery loses it’s charge if you leave it on the shelf a week?
Now I certainly wouldn’t want to run my lawn mower with such a battery. Even my car has occasional low-use periods like if I go on vacation. I’d hate to arrive back at the airport only to find I can’t drive home. But who says the same battery chemistry must be used for everything?
Shelf life, like other life span ratings doesn’t refer to simply losing charge via some discharge method. That would usually be a standby rating. Shelflife in this case may refer to electrolyte seeping out though surface pores, or a chemical degradation that is something that indicates whether actively maintained or not, you are going to have to replace the whole battery in a relatively short time.
The shelf life rating in many batteries is simply due to electrolyte drying out through normal venting. Once it is dry, ions can’t move so you can’t charge or discharge at all.
The aluminium battery has been the object of many scams in the past. Many people claim to have made a working one, but so far nothing of substance have come out.
Yeah, that was my first thought as well. A rechargeable aluminum battery with an aqueous electrolyte implies the ability to reduce aluminum compounds to metal underwater. Someone tell Alcoa about that, so they can stop wasting money on cryolite and furnaces. I’ll believe it when I see it in a product.
Reducing aluminum is no easy feat…I’ve seen ‘wonder aluminum batteries’ crop up since the 1970’s, but nothing ever seems to make production. ‘Radio Electronics’ magazine ran an article about the realities of re-charging such a battery…can’t be done.
Besides the weight and production footprint, longevity is one of the biggest factors to be considered in future storage solutions if EV’s are going to have a chance. If the chemistry is going to require replacement after two years, resulting in a cyclic investment that rivals the MSRP of the new vehicle, it just isn’t going to work; having “disposable” cars is kind of going against the whole point or EV’s in the first place. If you think E-waste is a problem now…..
A cheap totally recyclable battery is a decent aim though. Like $500 core charge and $500 every couple of years to swap it.
It’s also possible an in vehicle regeneration cycle is a thing with wet batteries, like drain, flush with weak acid to clean the plates, then refill with fresh electrolyte.
If these are coming up at less than IC car cost in oil changes or transmission flushes over a few years, they are worth looking into.
I agree that this doesn’t look like the future battery of choice for EVs, but think of all the AA and AAA batteries out there. Given it’s nominal voltage it could be a direct replacment, and if it’s cheap and highly recyclable that’s still a win.
It has to compete with batteries that use various nickel chemistries, in that case. For a lot of purposes, a low-self-discharge NiMH AA or AAA is too good to be worth replacing.
But if you’re a car maker a “disposable” car is a dream come true.
Rather than ‘dispose’, aluminum would be easy to recycle, no?
I think the point is that were the battery discarded, it would be less of a concern environmentally than any of the current technology. (Heh, see what I did there?)
Gmg already has an aluminum based battery
https://ca.finance.yahoo.com/news/gmg-achieves-initial-500-mah-120000631.html