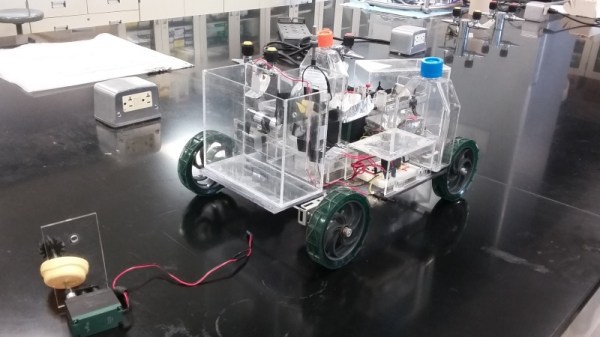
The race for chemical engineering is quite literally on. Every year, the American Institute of Chemical Engineers (AlChE) brings together hundreds of university students to face-off to design the fastest car using techniques they’ve learned from chemical engineering courses.
The Chem-E-Car competition races cars which are only powered by chemical reactions. The goal is to come up with an elegant solution – you can’t simply jettison matter out the back as the method of locomotion. In particular, the rules don’t allow the use of liquid or obnoxious odor discharge, commercial batteries, brakes, or electrical/mechanical timing devices. However, this doesn’t mean that electronics are absent from these designs. Many teams must gather data in order to design a control system to improve the performance of their car.
Students have to build a power system, stopping mechanism, circuitry, and mechanical assembly for the body of the car, all to fit in a size constraint not much bigger than a shoebox. The competition primarily judges the accuracy of the chemical reaction for stopping the car more so than speed or power. Given that the load the car must carry is typically unknown until the day of the competition, this is a significant challenge, allowing teams to find a way to design a flexible reaction that can accommodate a range of loads and distances.
For example, this 2015 entry from the Rice University team (PDF) uses a fuel cell for locomotion and an iodine clock reaction as a timer for braking. The fuel cell powers an Arduino which monitors a light-dependent resistor. In between the LED and that LDR, the clock reaction turns opaque at a predictable time and triggers the motors to stop turning.
While many schools choose not to disclose their designs in order to gain a competitive edge, we applaud the teams who have shared the story of their builds. Kudos to the Rice team mentioned above, to the 2014 Rutger’s team whose white paper outlines the construction of aluminum air batteries worthy of Walter White, to the car from the Universitas Negeri Semarang, Indonesia powered by a thermoelectric generator (PDF), the UC Berkeley team for outlining numerous approaches to developing their power system, and the two Ohio State team’s entries seen winning the regional competition in the video below.
If you were on a team that compete the the Chem-E-Car, we want to hear about it!
Continue reading “A Car That Runs On Homemade Chemical Reactions” →