As common as uranium is in the ground around us, the world’s oceans contain a thousand times more uranium (~4.5 billion tons) than can be mined today. This makes extracting uranium as well as other resources from seawater a very interesting proposition, albeit it one that requires finding a technological solution to not only filter out these highly diluted substances, but also do so in a way that’s economically viable. Now it seems that Chinese researchers have recently come tantalizingly close to achieving this goal.

The used electrochemical method is described in the paper (gift link) by [Yanjing Wang] et al., as published in Nature Sustainability. The claimed recovery cost of up to 100% of the uranium in the seawater is approximately $83/kilogram, which would be much cheaper than previous methods and is within striking distance of current uranium spot prices at about $70 – 85.
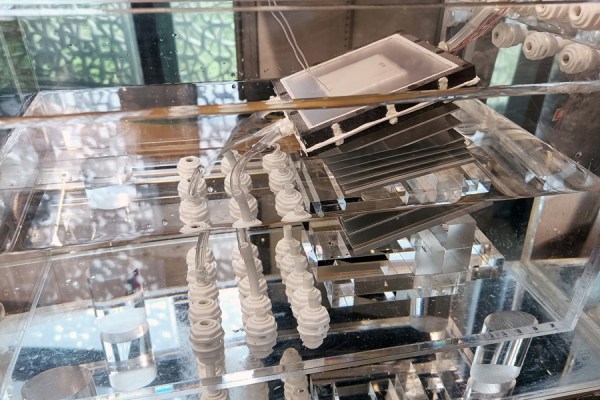
Of course, the challenge is to scale up this lab-sized prototype into something more industrial-sized. What’s interesting about this low-voltage method is that the conversion of uranium oxide ions to solid uranium oxides occurs at both the anode and cathode unlike with previous electrochemical methods. The copper anode becomes part of the electrochemical process, with UO2 deposited on the cathode and U3O8 on the anode.
Among the reported performance statistics of this prototype are the ability to extract UO22+ ions from an NaCl solution at concentrations ranging from 1 – 50 ppm. At 20 ppm and in the presence of Cl– ions (as is typical in seawater), the extraction rate was about 100%, compared to ~9.1% for the adsorption method. All of this required only a cell voltage of 0.6 V with 50 mA current, while being highly uranium-selective. Copper pollution of the water is also prevented, as the dissolved copper from the anode was found on the cathode after testing.
The process was tested on actual seawater (East & South China Sea), with ten hours of operation resulting in a recovery rate of 100% and 85.3% respectively. With potential electrode optimizations suggested by the authors, this extraction method might prove to be a viable way to not only recover uranium from seawater, but also at uranium mining facilities and more.