It’s no secret that fossil fuels are quickly becoming extinct. As technology charges ever forward, they are disappearing faster and faster. Many of our current dependencies on fossil fuels are associated with high-energy applications like transportation. Since it’s unlikely that global transportation will ever be in decline for any reason other than fuel shortage itself, it’s imperative that we find something that can replicate the high energy density of fossil fuels. Either that, or go back to the drawing board and change the entire scope of global transportation.
Energy, especially solar and wind, cannot be created all over the world. Traditionally, energy is created in situ and shipped to other places that need it. The proposed solutions for zero-carbon energy carriers—batteries and hydrogen—all have their weaknesses. Batteries are a fairly safe option, but their energy density is pretty poor. Hydrogen’s energy density is higher, but its flammability makes it dangerously volatile to store and transport.
Recently, a group of researchers at McGill University in Canada released a paper exploring the use of metal powders as our zero-carbon fuel of the future. Although metal powders could potentially be used as primary energy sources, the transitory solution they propose is to use them as secondary sources powered by wind and solar primaries.
There is Nothing New Under the Sun
The idea of using powdered fuel as an energy source is not a new one. One of Rudolf Diesel’s late 1800s prototypes ran briefly on coal dust, a resource which was plentiful in the mines of nearby Ruhr valley. After running the engine for less than ten minutes, he found that sludge had already accumulated and figured it was from the ash produced during combustion. Coal dust was tested further in Germany and the results were largely the same—internal sludge buildup and a higher rate of wear. Coal fuel research picked up in the US after WWII and focused on using a coal slurry made with diesel. Still, the ash caused the piston rings to wear out faster.
As the decades went on, diesel firms experimented with using smaller and smaller introductory coal particles, and they also tried mixing them with water instead of diesel. In the 1980s, the United States Department of Energy launched a program to work with diesel companies on the water-coal slurry question. One of those companies, a division of General Electric, had made big strides by the early 1990s, but by then, oil prices were declining. The DoE program was axed.
Modern Metal
Aluminium and other metals are an attractive alternative energy choice for a couple of reasons. Most importantly, they have a high energy density. That’s partially why aluminium powder is used in fireworks and rocket boosters. Metal can be used to make battery anodes, although metal-air batteries have to be much larger in order to compete with the power densities of traditional fuel cells. More resourcefully speaking, metal powders used as standalone fuels for direct combustion from the infrastructure level down to car engines.
There’s a problem with directly using metal powders as fuel for internal combustion engines, though. Much like coal dust and slurry, the combustion of aluminium and iron powder produces solid metal oxides. These oxides will coat the engine, wear it out faster, and eventually foul the pistons.
The irony is that those solid metal oxides are the key to renewability. They can be collected and recycled back into powdered fuel using existing infrastructure under wind and solar power. But the smaller they are, the harder they are to collect. And really, unless the recycling is efficiently carried out, metal powders aren’t really a good substitute for petroleum or diesel.
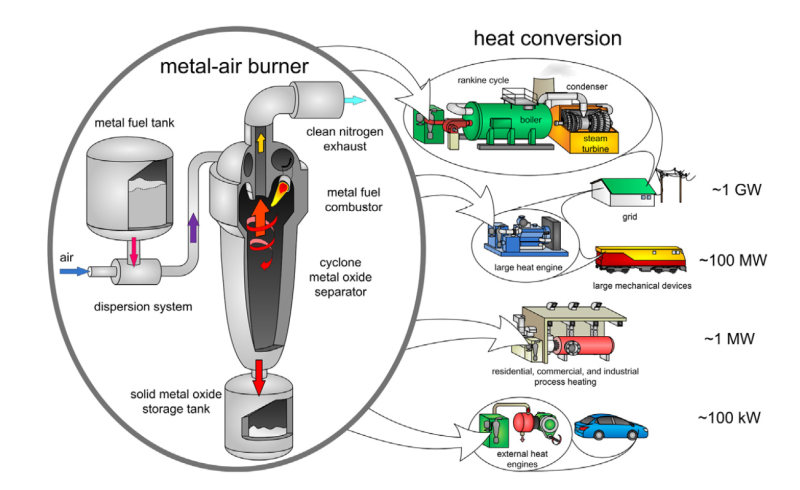
External combustion engines are a better application for metal powder fuel. The combustion system can do the dirty work at a safe distance. It can filter out the metal oxides with a cyclone and send only clean heat to the engine. Even more promising than aluminium is iron powder. It burns 1,000 Celsius degrees cooler than aluminium does, and it produces larger oxide particles that are easy to collect.
Although theoretically possible, a lot would have to change before we’re all driving (or being driven by) cars with, say, Stirling or steam engines that run on metal powder. Existing combustion schemes would have to be altered to support powdered metal fuel, and there would have to be a plan in place to collect everyone’s metal oxides for recycling. At the infrastructure level, nothing has been built that can convert metal powder at power densities comparable to fossil fuels.
Metal Powder Doesn’t Grow on Trees

It takes energy to make energy, and that includes making metal powder. Generally speaking, solid pieces of metal are either pulverized with machines, atomized with a stream of compressed air, or collected through electrolysis. Iron powder metallurgy in particular (Editor’s note: tee-hee) is typically performed using atomization or a reduction technique known as the sponge iron process. But these methods all depend on external energy. Atomization requires a gas, and the sponge iron process involves heating it up in a kiln to produce the intermediate sponge iron, followed by a lot of force to crush it into powder. At best, all of this processing could be done using renewable energy sources. At worst, it requires a fossil fuel.
Metal powder fuels alone won’t save us from our dependency on fossil fuels. No single type of alternative energy will. Most likely, it will take a combination of them working together to satisfy all the use cases before we can wean ourselves off of fossil fuels.
But if we make this shift, are we really just trading one non-renewable for another? Sure, iron and aluminium are fairly abundant, but we said the same thing about coal. Maybe once we’ve pulled all the metal out of the ground, we could last another couple of years or decades running on recycled oxides. Hopefully, we’ll have a new solution by then, like mining on the moon.
















No, seriously, the metal powder stuff is really dubious. It’s much more efficient to use organic fuels, which can also be produced regeneratively.
Capturing and recycling or re-concentrating the CO2 from organic fuels is a non-trivial problem as well. Though the massive existing install base for organic fuels means that organic fuels will stay the fuel of choice for most uses.
Imho, metal powder/slurry fuels get much more interesting when combined with metal-air fuel-cells. (aka refillable metal-air batteries) The metal-air fuel-cells open up the possibility of 50-80% efficient electricity-metal-electricity conversion with a fuel that’s easy to store and transport. I’d expect the primary use of metal fuels to be shipping solar power from desert areas to the rest of the world.
“Capturing and recycling or re-concentrating the CO2 from organic fuels is a non-trivial problem as well.”
Sure it is. Use cryo separation to recover CO2 from the atmosphere Convert it to CO and then use Hydrogen from water and the Fischer-Tropsch process to make synth fuel. All it takes is a lot of cheap power. I am thinking Thorium based fission or maybe polywell or lockheed high beta fusion.
Or just use plants.
Right. You wouldn’t use fossil fuels to make these metal powders, they’re supposed to replace fossil fuels. If you’re using external combustion, you can just use coal if you wanted to.
For all the energy it’d take, just plant some plants. Simple 1-step low-maintenance way of converting solar energy to something you can burn.
This metal thing might have some niche uses but it’s never going to power cars, or anything en masse. At least, as long as plants still grow, and we don’t have infinite free energy.
lockheed high beta fusion
fusion power is the incarnation of vaporware. Please.
YES! Burning organics is easy and as they are generally not electrically conductive they are difficult to use in a battery. And they great advantage is (up to now) that you can release the reaction products very easily into the atmosphere.
If you want/can not do this any more, a big advantage is lost. I think then the time is come to turn away from the inefficient heat engines and turn towards more efficient non thermal energy usage in batteries or fuel cells.
L.A.’s smog will come back with a vengeance if every car had a little campfire in it.
It’s called a plant!
This is definitely the future, I believe. Specifically biofuels. At some point, providing practical peak oil actually turns our to be a thing, or the environmental impacts are actually serious from burning fossil fuel, it will become cost effective to create bio-fuel, via algae. The best part is that the creation of the fuel pulls carbon from the atmosphere so it is effectively almost carbon neutral for those that care about such stuff. The other huge bonus is that our current infrastructure continues to work.
At the point of metal fuels, it’s be much easier and cheaper to build a Fischer-Trospch plant powered by hydro, solar, or nuclear, and split seawater for the hydrogen and use whatever renewable carbon source (lawn clippings, agricultural waste, corn currently used for ethanol fuels, etc.) as feedstock for the CO. Bam, carbon-neutral gasoline. (For diesel, of course, you can just lightly modify seed squeezin’s/used frying oil). Or feed the biodiesel into cracking reactors and turn it into gasoline … I guess Fischer-Tropsch is only really usefull when you’re pulling the carbon feedstock out of the air itself (which, while impractically expensive at current prices for distilled CO2, is probably not a bad idea.)
And by “useful” I mean “more economical that biodiesel”.
Whatever happened to that binary fuel tank idea that was in the running for HAD prize a year ago? Using calcium hydride to produce large amounts of hydrogen that then fed generators or what have you. Seems like calcium hydride would be a good intermediary storage technique since you produce it by recombining hydrogen and calcium under heat and pressure, not to mention it is stable if kept dry.
This sounds like yet another metal-hydride H2 storage system. I think there has already been research on some of this. Is the calcium-hydride version cheaper or better?
So calcium hydride is a chemical compound, visually indistinguishable from lime but when you put this powder in water it produces lots of hydrogen gas. Calcium hydride is converted to lime in this reaction and it’s not a metal hydride like storage solution as it cannot be recharged, but the plus side is that this reaction can produce larger amounts of hydrogen gas. And again it’s stable and not dangerous and all that.
Some dude made a smart fuel tank that monitored the output of hydrogen gas that it generated and added more calcium hydride to the mix to keep a steady stream of hydrogen that fed a small internal combustion electric generator. It was on hackaday.io but it seems to have vanished.
Betteridge law: “Any headline that ends in a question mark can be answered by the word no.”
“It’s no secret that fossil fuels are quickly becoming extinct.” ?!?!?
“Tech” writers will say literally anything.. Absolutely anything is possible to people who’s primary sources are other tech writers.
Maybe the writer is thinking of ‘Peak Oil’ – a concept now considered false, and possibly a hoax.
Most likely the whole of ‘peak oil’ was propaganda price-fixing from oil companies.
Peak oil
Actually, it haven’t been proved false. Only on the US soil because hydraulic fracturation raised the oil production and just postponed the prediction.
It could have easily been propaganda, but some article or other I read claimed that even without fracking peak oil is not and was never true.
Look up “abiogenic oil”. Oil pumped up from the deep wells, below many layers of impervious rock, does not show evidence of a biological origin. There have been some “pumped dry” wells that have refilled and become productive again, and the oil is different than was originally pumped out.
There simply has never been enough biomass on Earth to get subducted via plate tectonics and converted to the amount of crude oil that has been pumped since the late 1800’s and the known/assumed amount still in the ground.
But of course the “It’s gonna run out in 10/20/30/40/50/sometime years!” people and most of the big oil industry claim the concept of chemical processes deep underground are constantly producing oil is nonsense. If there’s an essentially infinitely replenishing supply, then the rationale for keeping the price up is gone.
4.6% of all meteors are carbonaceous chondrites and so that indicates that there could be a lot of very old carbon compounds left around from the formation of the earth, and moon. Add Polycyclic aromatic hydrocarbons to water under extreme heat and pressure, in the presence of iron or nickel oxides and they can reform into a huge number of different hydrocarbons.
Abiogenic oil is the ” 90s cold fusion” of the geologic world. It is typically reduced down to anomalous measurements and wishful thinking. Oil is typically made from plankton remnants being lithified, undergoing diagenises, and then concentrated to producible level by stratigraphic traps. Very rarely does plate techtonics play a role in the formation of the oil. A dry well will gradually refill overtime naturally from but transmissivity is insanely slow, as in mm/year in some source formations, this is combated now by fracking.
“…now considered false…”
Huh? Only if you’re a right wing climate change denying nutter.
From wiki:
“Oil production forecasts on which predictions of peak oil are based are often made within a range which includes optimistic (higher production) and pessimistic (lower production) scenarios. Optimistic[4] estimations of peak production forecast the global decline will begin after 2020, and assume major investments in alternatives will occur before a crisis, without requiring major changes in the lifestyle of heavily oil-consuming nations. Pessimistic predictions of future oil production made after 2007 stated either that the peak had already occurred,[5][6][7][8] that oil production was on the cusp of the peak, or that it would occur shortly.[9][10]”
It continues:
“However, a number of industry leaders and analysts believe that world oil production will peak between 2015 and 2030, with a significant chance that the peak will occur before 2020.[130] They consider dates after 2030 implausible.[131][132] By comparison, a 2014 analysis of production and reserve data predicted a peak in oil production about 2035.[133] Determining a more specific range is difficult due to the lack of certainty over the actual size of world oil reserves.[134] Unconventional oil is not currently predicted to meet the expected shortfall even in a best-case scenario.[131] For unconventional oil to fill the gap without “potentially serious impacts on the global economy”, oil production would have to remain stable after its peak, until 2035 at the earliest.[135]
Papers published since 2010 have been relatively pessimistic. A 2010 Kuwait University study predicted production would peak in 2014.[136] A 2010 Oxford University study predicted that production will peak before 2015,[10] but its projection of a change soon “… from a demand-led market to a supply constrained market …” was incorrect. A 2014 validation of a significant 2004 study in the journal Energy proposed that it is likely that conventional oil production peaked, according to various definitions, between 2005 and 2011. A set of models published in a 2014 Ph.D. thesis predicted that a 2012 peak would be followed by a drop in oil prices, which in some scenarios could turn into a rapid rise in prices thereafter.[137] According to energy blogger Ron Patterson, the peak of world oil production was probably around 2010.[12]”
Outside of the Oil industry’s lobbyists, the only real argument against us hitting peak oil seems to be that we might be on track to replace fossil fuels before we actually hit the peak. Which is an exceptionally dumb counter argument.
“If we continue to use this, we’ll run out.”
“Well, we aren’t going to keep using it, therefore your idea that we’d run out if we kept using it is wrong, because we won’t.”
No doubt peal oil is considered a hoax by those who don’t want to deal with the fact petroleum is a finite resource. Until the drop is consumed peak oil is going to be a moving target The actual point affected by new reserve discoveries/production, how bad or well or bad the economy is doing. When oil sales moved from the spot market to the commodity market 12-70 or so the oil companies haven’t had much influence on the price, not to the extent speculators in the commodity and futures markets do. The idea some have that oil companies can curtail production thereby affecting prices ignore that the companies have to honor lease contracts Most royalty owners don’t have the ability to do with that income for very long. Some lease stipulate that the well be produced or the well bore become the property of land and royalty owners.
There is a theoretical limit to oil fields, but we’ll all die off if we try to use all the oil available. Peak oil won’t be hit because we’ll self-limit the process, one way or another. Hopefully in the way that doesn’t involve a mass extinction event added to the geological record.
Fossil fuel ‘extinction’ is a political process, not a natural one.
Bring in the economists! There _is_ a finite amount of oil in the world, but it’s not the case that we’ll continue to use it at the same rate or price in the future. (And we keep discovering more fields and better means of extraction.)
There are fossil fuels all over the place, at varying extraction costs. The really deep stuff is more expensive to pull up, and so it’ll just cost more. When the extraction cost gets so high that solar/wind/whatever energy becomes competitive, we’ll stop using oil because it will no longer make sense. But that will happen _long_ before we actually run out.
The current fracking revolution is a blip, though. There’s overcapacity in the US at the moment, and firms are going bankrupt because they over-invested. Because of this, oil will not get cheaper forever, but we’re not “running out” anytime soon either.
Just as petroleum made whale oil too expensive!
Not to mention baby oil.
So there you go, powdered metals are not a source of energy, they are an energy carrier. Converting one form of energy into a less powerful substitute. Just like hydrogen. A dead end.
No. Hydrogen gas by itself is a bad in a different way.
It’s the smallest molecule that we know of. And it has a tendency to ‘sneak’ out of any gaskets, seals, or connectors you use to try to contain it. And then we mix the wonderful phenomenon of hydrogen embrittlement, where hydrogen diffuses into the container metal. The result is the container you thought was holding the H2 gas well…. isnt. And the resulting explosion is none too friendly either.
Better yet, is to use chains of hydrogens connected in between by carbon. Some of those types of chemicals are even liquid at standard pressure and temperature (octane).
Now, hydrogen is really awesome if one is to generate it and use it quickly (meaning no storage) . An HHO torch can be built cheaply and generate a very high heat flame. But it’s a very different usage outside energy storage systems.
>Better yet, is to use chains of hydrogens connected in between by carbon. Some of those types of chemicals are even liquid at standard pressure and temperature
That’s an awesome idea! What would make this possible is nanoscale robots that weld atoms together.
“Nanoscale robots” have been making hydrocarbons for nearly 4 billion years.
The oil refining process requires massive amounts of energy and materials to carry out as well. Simply because something requires a large input for a mediocre output doesn’t mean its a dead end. The benefit to oil refining is that in one batch process you get a plethora of different products. Plastics, pharmaceuticals, solvents, fuel oil, diesel, and the ever popular gasoline. All of these were products that evolved because of the refining process.
Who knows what kind of different metal complexes or advances in materials science scientists could gather simply by exploring this further!
>>Who knows what kind of different metal complexes or advances in materials science scientists could gather simply by exploring this further!
Anyone with a high-school understanding of chemistry who stops to think for a second.
Native metal + oxygen ==> metal oxide + energy
Always.
There’s no way around this.
Refining oil is in no way similar to oxidizing metal. The reason we get so many products from oil is because crude oil is a mix of dozens of compounds with ,on average, 5-40 carbon atoms alone. Never mind that each string of carbons is part of a complex molecule in its own right.
Metal fuels are fighting pesky thermodynamics the whole way, while fossil fuels are coasting downhill from the second wet pull them out of the ground.
It sounds like the metal fuel would be better served by trying to replace/augment a coal fired power plant first to get the kinks out of the process, then scale it down to cars and other consumer goods.
Although this raises a question I’ve had for some time: How hard is it to create new petroleum from organic waste without using geologic processes and time scales? In other words could we take a bunch of organic waste from a restaurant, put it in a pressure cooker*, and convert the waste to oil**? How about all the fat removed from liposuction? Could that be converted into oil**?
*An industrial one, not a stovetop model.
**Or oil precursors.
BtL processes generally need a whole bunch of energy. You can turn wood into diesel at a loss of 50% of the energy. Stuff that’s already oil requires less energy.
The conversion isn’t really the problem, but the lack of feedstock.
Maybe, but the carbon in plants came from the atmosphere in the first place, so it’s good at keeping the CO2 level down. We just need some fast-growing plants, maybe work on converting bamboo into fuel, or of course algae.
https://en.wikipedia.org/wiki/Thermal_depolymerization
>Thermal depolymerization (TDP) is a depolymerization process using hydrous pyrolysis for the reduction of complex organic materials (usually waste products of various sorts, often biomass and plastic) into light crude oil.
>As reported on 04/02/2006 by Discover Magazine, a Carthage, Missouri plant was producing 500 barrels per day (79 m3/d) of oil made from 270 tons of turkey entrails and 20 tons of hog lard. This represents an oil yield of 22.3 percent. The Carthage plant produces API 40+, a high value crude oil.
IMHO this and related gassification/GtL fuel processes deserve much more attention then they get.
Iron is not the right energy storage/carrier if you want to stay away from carbon emission. To recover iron back from its oxides, you typically use carbon based fuel as a reduction agent.
“For Integrated steelmaking, the primary sources of GHG emissions are blast furnace stoves (43 percent), miscellaneous combustion sources burning natural gas and process gases (30 percent), other process units (15 percent) and indirect emissions from electricity usage (12 percent),” the report said, estimating that the U.S. steel industry produced 117 million tons of carbon dioxide in 2010.
GHG = Green House Gas.
Using aluminum welding wire underwater to produce hydrogen which then powers the engine. The aluminum oxide can be recycled. http://www.google.com/patents/US4702894 Neat idea.
“Although metal powders could potentially be used as primary energy sources”
Unless you find a lump of pure iron or aluminium in the ground, no you could not.
It’s not like you go ahead and dig up BP gas stations buried under the ground. Oil needs to be refined and processed, too. The only question is whether you can make the mining, refining, and processing less than the energy released after burning.
“The only question is whether you can make the mining, refining, and processing less than the energy released after burning.”
Which in the case of metal fuels is a very solid ‘no’. The problem is that most metals aren’t simply refined, but smelted. They must be chemically extracted from their ores, and by burning iron or aluminum you are basically reconstituting the ore. It’s like using energy to extract hydrogen from water and then burning the hydrogen to get water and energy. There’s not net gain.
You can’t.
Energy has to be put into raw (oxidized) metals (thus reducing them) before processing them into powders for convenience of use. Oil is already full of eneregy, even though we have to process the oil to make it more convenient. When the metal powders are burned (oxidized), less usable energy is captured on release than was used to construct them. This is also true of oil, but we didn’t have to expend energy to construct the oil from [oxidized] precursors (CO2) because organisms did so for us long ago.
Metals exist in the groud in the form of minerals – oxides.
Oil is a net energy source. Mineral ores are not. Metals are not a primary energy source unless you happen to find a pure chunk of it.
https://en.wikipedia.org/wiki/Scuttling
>Ships are increasingly being scuttled as a method of disposal. The economic benefit of scuttling a ship includes removal of ongoing operational expense to keep the vessel seaworthy.
That’s a good source of metal…
That’s a terrible waste of ship.
Not really. A lot of these ships are sunk to create marine habitats that help in the recovery of coral ecosystems in places that have been damaged. They are also great dive sites.
A lot of them? Much much less than even a tenth of a percent of all scuttled boats are done to create marine habitats, or do anything positive.
Are there any pure metals found in the ground? Excluding gold and silver, I suppose. If you can dig it up in pure form, it’s obviously not that reactive with air, so a bad idea for a fuel.
It’s relatively easy to remove metals like magnesium from seawater.
Yes amazing idea, we should also put gerbils in wheels and have them run to power our cars, and then we can also get really big rubber bands and wind them up before hand to turn a mechanical crank.
I’m pretty sure that somebody did try to make a big spiral wound clockwork car back in the day.
Flywheels! We need more flywheel-powered cars! Added bonus: it’s impossible to roll the car over. You might not be able to take any corners either depending on your spin axis but hey it’s a small price for such a clean and cool solution…
SciFi novel WINDUP GIRL.
If we are to use metal powder fuel we should use lead and mercury. (Sarcasm)
Well, if we use mercury we can just squirt it in like gasoline!
Problem solved!
We already did lead.
Don’t hassle him, he’s the inevitable result of our previous use of lead in fuel.
Just grow some algae in vats and convert it into crude oil with high pressure and temperature. Every other alternative is too expensive, too complex or too unreasonable…
It’s a shame that nobody has gotten the system you describe to actually be cost-effective yet. At some point, hopefully before there are no remaining fossil fuels, it will become so.
I should have also said that every other alternative in use is currently cheaper and simpler. Unreasonable or not is a judgement call.
Actually fuel synthesis was cheaper when crude oil costed more than 60USD/barrel. Bio-fuel synthesis requires new infrastructure to work at industrial scale. Both RPA and Nazi Germany used older conversion technologies to produce industrial quantities of fuel. With algae it is at l;east ecologic, because carbon dioxide is consumed by them while creating more biomass. They don’t implement it yet because we have oil for now, To make algae fuel cheap enough we need to increase production of it, or wait until oil becomes more expensive…
That is very interesting. Do you have some good references for the economics of algae-fuel?
No one has worked out the details for making algal farming cost effective even at $100 oil. But it’s clearly the best option. There are major ($100 million) pilot projects that have been announced. It uses photosynthesis to remove CO2 from the ecosystem and combine it with H2O. When it’s burned it goes back to what you started with.
Most of the effort I’ve heard about is focused on genetic engineering of algae to have a cost effective composition. The natural formation of oil and gas from organic matter is very inefficient and slow.
Metal dust as fuel is simply silly unless someone manages to find or create an organism which consumes rust and excretes iron at effective efficiencies.
One man in Poland started algae farm and made fuel from it that was cheaper than commercial one. Unfortunately for him polish law makes it illegal to produce and use or sell fuel made at home. I read quite a bit on this subject few years ago…
“Poland man finds a trick to make cheap fuel. Oil companies are mad at him.”
Actually government was mad at him because he could avoid paying excise tax. So instead of changing tax law they outlawed alternative fuels. They also changed Renewable Energy Sources law in such a way that it’s illegal to build any wind turbines on the 99,9% of surface of Poland. And remaining 0,1% is not very good place for such turbines…
Photosynthesis ~1% conversion efficiency
Photovoltaic panel – up to 22% conversion efficiency
Direct heating – even more, depends on how much you want to pay
Do you now see why growing plants as fuel is silly? For food – sure, we can’t eat alkanes (a pity :D), but as fuel it makes little sense as the piss-poor conversion efficiency means you’d need a much bigger used surface.
If you’re going to do high pressure and temperature, you might as well feed it with a different hydrocarbon source, like common trash. Plenty of that to go around and it will have exactly the same technical problems as algae (inorganic residues), thus there’s no need to grow algae. Also, the inorganic trash residue can be processed for things like precious metals.
Isn’t most carbon-bearing trash going to be food? Which comes from photosynthesis. I dunno if there’d be enough food waste to create enough petrol. What isn’t from food would be plastic, from oil. There’s already power plants that burn rubbish, probably the best way to use it. That, and methane deposits in old landfills that built up while nobody was paying attention.
Plants have a much lower setup cost than photovoltaic panels, which need a lot of energy to produce the pure silicon they’re sliced from. Plants are also suitable to, er, plant, in all sorts of places. Low maintenance, self-replicating, probably the reason we don’t produce carbohydrates in gigantic factories.
Any combustion energy scheme that is going to require a vast new infrastructure with backhaul built in is stillborn at this point because initial costs will render it non-competitive.
Can 100% of the oxides be recovered? I’m imagine aluminum oxide coming out the tailpipe. 10 years later Alzheimers rates are rediculous high!
Ok, a quick Google showed me that link hasn’t been proven and is quite likely false. That doesn’t make it healthy though. I’m pretty sure that metal oxide exhaust would turn out to be even worse for us than the half-burnt petrolium we are all inhaling today.
Aluminum Oxide is corundum, a very hard solid… you’d basically get a fine sand coming out of the ‘tailpipe’.
Also a very bad thing to have in your engine.
And your lungs
And that is a key point, particulate pollution is deadly, it is just that it kills you slowly.
I think the point is you don’t burn it in your ICE, you have a reactor designed to collect the Oxides.
4kg Al + 1kg O2 –> 5kg Al2O3 , then reverse the process with your *bff* renewable electricity to recover the 4kg of Al
It turns the metal powder into a *battery* that doesn’t really wear out (assuming you capture all the oxide “sand” and stores nice and stable.
Heat from the reactor powers a sterling engine (as the article says)
Aluminum and Oxygen are two of the most abundant elements on earth. And aluminum loves oxygen. So most Al is lying around in oxide form. It is very inert and generally non toxic. Except fine powders in your lungs, this is valid for many forms of fine dust, but this has nothing to do with alzheimers disease, which is a brain disease, nut lung disease.
And the idea is to recycle this oxides anyway.
In the 60s-70s, there was some concern that Aluminum exposure was important in the development of Alzheimer’s, but more recent research has ruled out any connection. The idea of the connection is still circulating, of course, because [internets].
Aren’t there aluminium plaques found in the brains of Alzheimer’s patients? Humans need no aluminium at all to live, and it has to get in there somehow.
I haven’t thrown out all my aluminium pans, not being a “health” nut. Is the plaque thing not true?
They are also found in non – Alzheimer’s patients.
“in situ” means on the site, “the” implicitly referring to some context, and the only site in the context is “other places where it is used”. So you should write “ex situ” instead.
I see this every five years or so, presented as if it’s a new idea. But never any new ideas or progress. Or even any decent information on which to evaluate the claim.
Let me attempt to provide the latter.
According to Wikipedia, “Steam engines and turbines operate on the Rankine cycle which has a maximum Carnot efficiency of 63% for practical engines, with steam turbine power plants able to achieve efficiency in the mid 40% range.”
Efficiency goes down with scale. So let’s say a steam turbine scaled down to a size appropriate for a car is only 30% efficient (and that may be overly generous).
Best I was able to determine, the amount of energy released from oxidation of iron is 489MJ/ton. The amount of energy to recycle that oxide back to iron in a blast furnace, based on worldwide average of real furnaces, is 11.6GJ/ton. So providing the original iron, or recycling it (same thing as iron isn’t found free in nature) is only 4.2% efficient.
Between these two losses, we’re already looking at only 1.2% of the energy going into this actually providing motive power for your car. But additional energy losses will be incurred in moving the new/spent fuel back and forth to processing plants. Powdering the iron fine enough for efficient burning. And so on.
An energy storage method less than 1% efficient sucks. I was able to determine that in a mere 10 minutes. The McGill researchers either could not, or have chosen to ignore it. And are instead trolling for funding to build a prototype burner, when the burner is not even close to being the biggest problem with the whole process.
the amount of energy released from oxidation of iron is 489MJ/ton. This number does not seem to be right. The heat would be about 7GJ/ton.
To make an efficient heat engine with metal, you would need:
elemental al, and non-oxidized steel or iron powder.
You could start out burning iron powder, and turn it into an oxide(net gain of heat). Then you would take the iron oxide and mix 50/50 with the aluminum powder to make thermite, and re-burn. Don’t know if the iron would be hot enough to start the thermite reaction. But it would have to be small reactions, as thermite in mass will destroy stuff. Not very explosive, but should be able to boil water with enough thermal mass to sustain the water boiling. Might be good for home heating, or steam production. If there was a way to keep the iron mostly separate after the reaction(like a water bath), you could re use it as fuel(Although at the point of diminishing gains).
What chall think?
The biproducts would be iron oxide(re burnable), aluminum oxide, and iron entombed in aluminum oxide(not efficiently usable afaIk). Again,the reaction is slow, but has a large amount of heat, which rules out jet or piston engines. But there is a huge gain of heat.
With all the talk about energy required to refine powders or other alternative energy source, you’re all missing the point. The point is, Global Warming, and Limited availability. If we accept that global warming is a man made situation caused the release of organic compounds in the air, then the fix is to use a power source that no longer does that. Further, oil will run out, because it can’t be produced by natural process faster than it is being used. Given that the powdered metal solution is better for both these reasons, lets look at net energy.
So, While lets say yes, it takes 100X energy to produce/recycle aluminum powder than it does to convert crude oil into gas. It’s not about that loss… it’s about the portable power unit. The article seems to indicate that the powdered metals can release a comparable amount of energy per unit to oil based products. Batteries can not… not by a long shot. The power used to generate the the aluminium powder could be solar, geothermal, or wind… something that does not have the instantaneous power potential but does have sustainability. The medium itself is the storage of that reusable energy source into an instantaneous high energy source. Is there a net loss over oil? Certainly. but that net loss is of an energy source that is infinite and not harmful to the planet. So who cares?
Will it cost money? Probably a shit-ton. But that’s only because the people with money and power are more interested in money and power than the survival of the species.
I think this is a brilliant idea and should be explored further. Unfortunately there are relatively few external combustion engines that are as easy to maintain as a diesel engine, but that too will improve with a more widespread use of the tech, as there is currently no money in improving the efficiency or ease of maintenance of a Sterling engine. Once they become the primary engine type, there will be. Otherwise we’re back to steam engines, which did have a pretty good run, but in general are noisey, maintenance heavy, and messy… but maybe these can be fixed too?
The problem is that the energy needed to regenerate the aluminum is so massive compared to what you would get out by burning. It would be better for the environment to keep driving a fossil fueled car!
Especially since fossil fuels are responsible for generating most of the electricity in many countries.
Also see the comment by Chris C. above. Even if you only use hydropower or such to regenerate the aluminum, it’s still a terribly inefficient storage medium even if it is energy dense.
A better solution is to kick the radiation fear mongers in the ass and start building new nuke plants again (instead of constantly prolonging the life of the ancient ones) and developing better, safer and more efficient fission reactors for electricity production.
That alone would free up a considerable chunk of the fossil fuels to used elsewhere. Once we can deal with the largest polluter and CO2 source, then we can focus on the next. Not the other way.
Radically new nuke plant designs could also directly power chemical conversion processes (ammonia production for example), but that requires a crap-ton of money in research.
We have yet to prove if fusion is economically viable for anything else then bombs. If it is – great. If not – there’s enough fissible material to run literally for centuries. That should give us enough time to come up with a better solution.
The other, equally (maybe even greater) magalomaniac concept is renewable power on a stupidly huge scale with massive distribution networks around whole continents, since there’s always sun shining/wind blowing somewhere in the world.
Your first idea is better, nuclear fission is our future – just not everyone understands this yet. Wind and solar, good storage, good interconnects or not, will always suffer from the problem of scale because they are converting low density sources and if you build them big enough to overcome this limitation, there are going to be environmental consequences. Just look at the damage caused by the one renewable mode that is economically viable – hydro. Consider the size of those given high potential energy of falling water and the relative ease of converting it to electric power, and think of how big a wind installation would need be to equal it.
The bottom line is that it is nuclear, or the status quo like it or not.
renewables provide more than enough power for quite a few Scandinavian countries(as in with good wind, windpower alone produced no less than 140% of the danish concumption, though that is a bit of a trick of measuring, high production and low consumption, the average coverage is around 45%), not at a guaranteed level, a bad day of very high wind can force turbines to shut down over a large area, the wind is never still over the entire range of the current distribution networks so that is less of an issue, combined with solar it works fairly well already.
i agree that fission is a great technology but as i see it many of the other benefits of fission plants (especially research reactors) as being far more valuable, medical isotopes, radiation sources for imaging quite a lot more, combined with the inherent safety of many research reactors, some of them are so safe that they may run without supervision over night.
Renewable supporters love to quote great success for these technologies by including hydro, and indeed hydro is a successful renewable, but without hydro for spinning reserve wind and solar need thermal back up. Wind and solar are not significant sources that can stand on their own, and never will be, thus they are never going to be a way off combustion – ever.
Hackaday? WTF?!
Ah yes, external heat engines. Let’s just pretend we can make small efficient ones with power suitable for a car. All we need to do is pretend that steam locomotives aren’t just 6% efficient and only really the massive steam turbines in power plants approach any decent efficiency.
Right, let’s just burn metal powder and use the heat from the liquid metal result to power everything, that will be usable. HA.
Really, for a vehicle application if fossil fuels are not an option, redox flow batteries and regular stored electricity is more efficient, convenient, safer, easier to work on, easier to design, easier to build, &c. Powdered metal as fuel is a terrible idea.
Yes!
I hate to bring up physics but… isn’t it important to have as much energy per atomic bond, per mass of molecule, as possible therefore going with heavier elements such as iron is dumb? Isn’t this why hydrogen and hydrocarbons are smart, particularly in fuel cells?
Since at least 2008, there have been multi axis robot arms capable of lifting and moving up to 5,000 pounds. That’s plenty more than a typical string of drill pipe that’s three pieces screwed together.
In the first season of “Black Gold” there were three oil rigs. One dating almost back to the end of WW2, a bigger one several years old, and another that was essentially identical to the one built in the 1940’s. Aside from the power tongs (a large clamp with hydraulic driven rollers to screw and unscrew drill pipe) and various advances in drill bit configuration and materials, there’s been next to zero advancement in land based drill rig technology since the invention of the blowout preventer. None of those three rigs were using power tongs. They used the ancient pull chain. A roughneck swings a chain at the pipe so that it wraps around and grips the pipe. Then a winch pulls on the chain. Screwing or unscrewing is controlled by which way the chain is thrown around the pipe. Get the chain too short and it will pull loose, flying free. Roughnecks have been maimed and killed by those chains. Why any rig owner still uses the chain??? Why do rig manufacturers still offer the pull chain as an option on new rigs?
So where do robot arms come into this? The reason for the tall derrick is for handling the pipe strings. To speed up operation, pieces of pipe are screwed together into strings then stood on end and placed in a rack in the derrick. A roughneck has to climb up in the derrick to attach lifting cables to the strings and manhandle them out of the rack as they’re positioned above the rotary table to be connected. It’s very dangerous. Rougnecks have fallen to their deaths or get arms and legs smashed by stings they’ve lost control of.
The pipe could be added (or removed when pulling it up) one piece at a time, but that would triple (or quadruple for taller rigs using 4 piece strings) the time spent driving pipe.
So take a typical drill rig and cut away everything above the deck and rotary table. Add a simple tower with a robot arm on top. On the deck have another robot arm with a power tong gripper. About halfway up the height of the tower, place a horizontal drill string rack and a third robot arm. At the 1/3 and 2/3 levels place guide clamps which can open and close to receive the string.
To add a string to the drill, the middle arm grabs one at its center then rotates it upright, while the top arm reaches out to grab and steady the top end. Together they place it into the guide clamps, then lower it down against the pipe held in the rotary table. the bottom arm grips it with its power tongs and screws it together.
A simpler rig, built using much less steel. All weather, 24/7 operation. Steady speed of operation with no risk of injury to humans. It might even be possible to beat the speed of a human operated rig with robots running one piece of pipe at a time. With the lighter loads they could lift and screw pipe much quicker than multi piece strings.
Any way an automated drilling rig would be built, many of them could be monitored from a central location. If there’s a problem, try to fix it remotely. If that can’t be done, then call in the roughnecks to fix it.
So, at what point does the cost of human labor, especially high given the dangerous nature of the job + plus various types of insurance for the squishable humans, make the R&D and investment in the first robotic drill rig worth it? Once the first one proves it’s up to the task, many more should follow. Since the alterations would only be above the deck, I’d expect there to be a large number of conversions over all new builds.
This may be true, but has not much to do with the problems described here. The artcle is not about more efficient drilling but of replacing oil. Because some NGOs and politicians believe it is bad.
And almost all scientists who are experts on the subject, if we’re talking manmade global warming.
Follow the money. It’s not at all what we expect. Whatever delivers the best market product (currently petroleum) and delivers the highest profit (also petroleum) it will be the energy source utilized by the world. We’re not a victim of philanthropists and magicians in our daily lives, but by what best serves our needs and provides a living and profit to the greatest number of people. Reciprocal economics.
Oh, and while we’re on the energy subject – DOWN WITH ETHANOL!
Hopefully in the form of a good drink :-)
Burning Oil Creates CO, CO2 and H2O and Sulphates Plants love that stuff. They fix the Carbon and release the Oxygen.
If we start Burning Metals for fuel we will end up with Metal Oxides, Poisonous, Carcinogen dust.
And No Oxygen
So our proposed solution for running out of fossil fuels is to start using up our breathable air?
Seriously guys..
Combine the best of liquid fuels & metal powders: mercury.
Guys, youre lame. Biofuels are horrible you can regenerate them kinda, but think about emmisions from these fuels they will pollute your environment, create smog which is big problem for health of people. Yes you can somehow capture back these pollutants but people will be exposed to them before you can capture them. Just go inhale your car exhaust, thats whats bad for humankind, all of it. Not just co2. All of it. That fumes are same with biofuels. And every year there is bigger and bigger demand for transportation that means more and more fumes, smog, and small particles which can and will cause cancer. So we should think to move to fuels which have more then one good feature/characteristic, about cleaner fuels in every way. Not just making poems about co2 neutrality. Co2 neutrality is only short term goal which we really need to address but still only short term goal. We have to have really green/clean fuels.
Forget metal powders: if you’re going to use metal [use boron wire](http://www.eagle.ca/~gcowan/boron_blast.html).
The big problem with combusting any metals as a fuel is that you need to keep the Nitrogen away. They typically burn so hot that they tend to create even more of a NOx problem than really high pressure ICE’s do, even when burning in a (necessarily-cooler) continuous furnace.
Boria has the nice advantage in that it tends to collect itself into a liquid, which then can be easily frozen solid and pelletised (think: like icecubes) for easy bulk transport.
But it requires a completely 100% new infrastructure to handle it.
Plants have the problem that there’s only so much arable land to go ’round, so if you want more, you’ll need some very large desalination plants to get sufficient fresh water. They’re also, as others have said, relatively inefficient.
Renewables can and should displace most large-scale combustion base load, if things like LFTR’s and Thorcon don’t. Either way, we desparately need to find a way to make mass production of CO2 in relatively inefficient (yet, *cheap*) giant furnaces un-economic.
The dilemma is that mass energy storage is itself hard enough to make even concentrated solar difficult as a base load supply. However you do it, you have to trade monetary inefficiency (having lots of excess power collection capacity) for robustness ( the ability to be *certain* that base load can be met, rain, hail *or* shine).
The problem is that most large-scale spending is so narrowly focused on showing stakeholders that they’re managing their efforts with high efficiency *now*, that they can’t see that robustness against disaster is its own form of insurance.
In the case of solar concentrator power, one needs a worryingly large factor of excess capacity, because of weather and the relatively short duration each day that solar isolation exceeds half its full power level, even with clear skies.
Comparing to the dependably of combustion as a heat source, it’s just not reliable enough for those who must demonstrate their own “decision making efficiency” to their stakeholders, to fully rely upon. [And so they don’t](http://euanmearns.com/el-hierro-renewable-energy-project-end-2015-performance-review-and-summary/).
The problem might not be the fuel but the vehicle(not only the engine). I really think that fossil fuels will stick around a bit more. BTW most alternative fuels aren’t as green as they look at first sight…