Researchers have built a prototype lithium-sulphur battery that — when perfected — could have up to five times the energy density of current lithium-ion devices. Researchers in the UK and China drew inspiration from intestines to overcome problems in the battery construction.
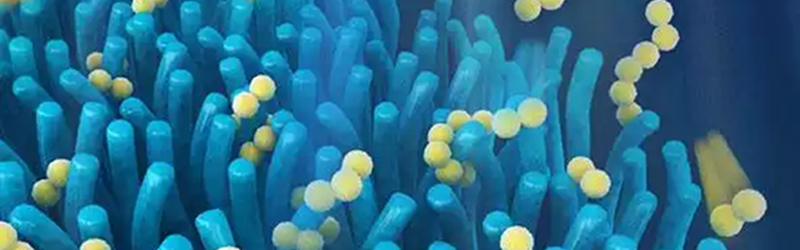
In your intestine, small hair-like structures called villi increase the surface area that your body uses to absorb nutrients from food. In the new lithium-sulphur battery, researchers used tiny zinc oxide wires to form a layer of material with a villi-like structure. These villi cover one electrode and can trap fragments of the active material when they break off, allowing them to continue participating in the electrochemical reaction that produces electricity.
Lithium-sulphur batteries aren’t new (in fact, they were used in 2008 in a solar-powered plane that broke several records), but this new technique may make them more practical. You can see a video about ordinary lithium-sulphur batteries below along with more on how this research improves the state of the art.
A typical lithium-ion battery contains graphite and lithium cobalt oxide. Positively charged lithium ions move back and forth from the cathode, through the electrolyte and into the anode. Since the carbon atoms in the graphite can only take (at most) one lithium ion, that sets the theoretical limit on how much energy you can draw from the battery.
Sulphur and lithium react differently, via a multi-electron transfer mechanism. That’s why sulphur can offer a much higher theoretical capacity. However, as the battery undergoes several charge-discharge cycles, bits of the sulphur break away causing the battery to gradually lose active material. The zinc oxide villi tend to trap these pieces which slows the degradation of the battery.
The villi improved the number of times the prototype battery can be charged and discharged, but it is still not able to match a conventional lithium-ion battery. On the other hand, the new battery doesn’t need recharging as often.
We’ve seen nanotech in improved batteries before. There’s also other research going on with using other materials with lithium.
Photo credit: [Teng Zhao]














Where would we be without Zinc Oxide!
If it works out, that skit from Kentucky Fried Movie will have a new wonder use for it.
Ah todays astonishing, new revolutionary battery technology that will Change Everything that at best is years, if not decades from a commercial product if it goes to market at all.
This is rather like the Li-Ion batteries with [gel-coated nano-wires](http://pubs.acs.org/doi/abs/10.1021/acsenergylett.6b00029) thing that was published a little while back. 100,000 cycles from a standard Li-Ion battery is nothing to snuff at, but it isn’t likely to be something we see in standard lithium cells anytime soon.
Oh look, this novel cell is old news, now we are waiting for brass-steel(!) batteries. https://www.eurekalert.org/pub_releases/2016-11/vu-mhb110116.php
It’s an improvement for mechanically damaged batteries but I do worry about it burning as a result of overcharge or thermal reaction. What kind of gas will it emit and what will be in the smoke? It would be really bad if it’s sulfur but not a dealbreaker.
I think that when you said “when perfected” your finger may have slipped and resulted in a slight typo. Of course we realise that you meant “when they have done the other 99.9 of the work necessary to create a commercially viable and consumer ready technology that meets the myriad of other requirements for a battery technology that can’t just summed up in energy density, such as cycle life, ageing characteristics, power density, thermal stability and manufacturability, having invested 10 years and billions of dollars”
Don’t forget that by then, as a result of all these public malfunctions with cellphones and e-cigs there will probably be some kind of draconian battery laws regarding the years of product testing and millions of dollars in impact assessments that will be required to change the color of font on the log of the thing.
Is anyone else worried about the ‘other side of the fence’ regarding the five times energy density? I mean, look at how much of an issue the current lithium batteries are causing with the Samsung Note 7… what sort of fun would we be having with batteries the same physical size but 5 times the electrical capacity?
That being said, it will be nice to have the larger capacity in an equivalent space, I just hope that whomever designs around it is really careful about how it’s used. I’m not sure we really need more exploding devices is all.
The video claims that Li-S is safer than Li-Ion. It includes a quick demonstration of a needle piercing a Li-S cell with no effect, followed by the same needle piercing a Li-Ion cell and starting a fire, so it may not go all Samsung on you.
Sulfur is generally less toxic to humans than the cobalt used in Li-Ion batteries, (the effects of cobalt are probably worse when inhaled in smoke) so it also has that going for it.
But yeah, anything that stores energy is usually capable of the rapid release of all that energy at once. They didn’t show that in the video.
although i agree with you about the energy-density being correlated with overall worst-case danger, it is not the reason as to why the S7 batteries went bad. there are already batteries with a higher energy-density then the batteries used in the S7.
as for the S7 itself, it could have been bad batteries, bad hacker/virus, bad charging/discharging, or even possibly other outside factors, such as sitting on the damm thing, (if the screen cracks, battery is flexing) or forgetting to RTFM, esp. where it compares useage-temp-range VS recharging-temp.-range. or is samsung just like all the other brands the last 10 years or so that wont tell us this? (no longer printed on paper) what about a warning about letting the phone warm-up (or cool-down) for at least one hour before charging after going from one climate to another? LIKE THAT INJURY TO AN ELEMENTARY-SCHOOL KID RECHARGING HIS APPLE DEVICE TWO MINS AFTER COMING IN FROM FREEZING RECESS. yes, even apple (consumers) have to obey the law(s) of datasheets!
cellcharging outside or right after extreme temperatures used to be non-existant (cept for hackers), now you see it everyday, EVEN IN SCORCHING AND FREEZING WEATHER, wonder if this has any effect on the batteries that expressly forbid this due to temp ranges in the product-datasheet that the average user will never read because he does not know which of the several models of battery are in his specific sub-sub-revision andor day-of-the-week-shipment of cellphone. there is only one hint aside from ruining the warranty to check, the IMEI, good luck asking the manufacturer directly…
and as for “HoverBoard” ?
reseller giving huge discount = knockoff or see above
That could go two ways… reduce the battery size by five times, or increase the effective run-time of the phone by five times. In reality it would be somewhere in the middle, so an S7 would probably on go off with something like twice as-big-a flame…
Except of course that Li-S is safer than Li-Ion, so there would probably be no flame at all.
A li-Ion battery has an energy density of 150Wh/kg. Gasoline has an energy density of 12kWh/kg.
BTW, there is this startup called Seeo which was bought by Bosch because of their “new” Li-Ion tech. Solid state Li-Ion battery. Instead of using the same old liquid electrolyte they’ve used a solid one. Judging from the product page of Seeo, those batteries really can take a beating without bursting in flames. And they have double the energy density as regular Li-Ion cells.
What ever happened to the printed lithium nano-particles with propylene-glycol? It’s solid at normal pressure and liquid under high pressure. Allowing batteries to printed. It’s energy density was 10x…where are they?
All this negativity, what about coming up with suggestions to help the designers of this battery get it off the ground my suggestion is that the cell makes use of having the maximum surface area to get the best out of it If my memory serves me well, somewhere on tut internet researchers have had the same problem with solar cells, and they used nano tech to etch a 3d structure onto the silicon to make it absorbs more light increasing the surface area and the efficiency by a factor in available with out using a 3d nano structure. This factor increase eludes my memory, but if I were the researchers of this LiS battery, I would be turning my attention to thus way of increasing the surface area of the anode\cathode . a theoretical increase of 5 over Lion\Lipo would be good, but an increase of 20 or 30 would be a quantum leap, I cannot wait. Hope I live to see it revolutionise many things.