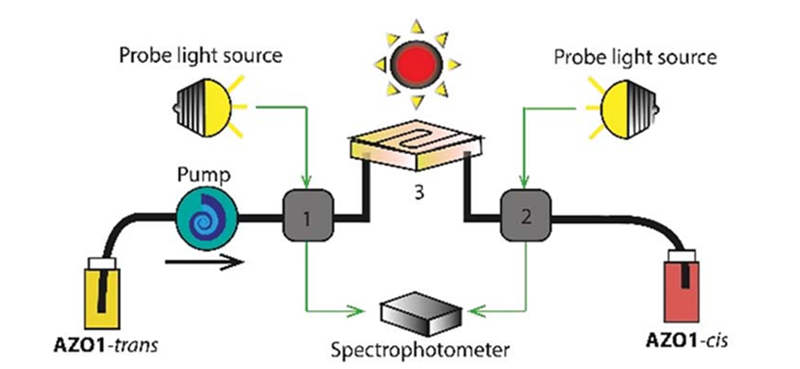
Probably the most efficient way to convert solar energy into electricity is the old fashioned way, heating water into steam and turning a turbine. This remains a messy affair though and you don’t really want a steam boiler on your roof, so solar cells are popular. However, there’s some new research showing how a molecule can absorb solar energy, store it, and then release the heat on demand years later. This could offer new ways to collect and even transport solar power. This new molecule, derived from azobenzene, holds immense promise to change the way we work with solar power.
The idea behind the system is that a parent molecule is isomerized by solar irradiation. The resulting photoisomer is stable for a long period. Adding a catalyst or heat will cause the photoisomer to revert back to the parent molecule, releasing energy in the process.
We will admit, we aren’t chemists so some of the paper was a bit over our heads. But the basic idea is appealing, and this sounds like a field where a garage chemist might be able to contribute. Perhaps one day the desert will be producing photoisomers in the same way it currently produces petroleum.
We always thought the future of fuel might be biofuels, but maybe it will be solar, instead. Maybe the future solar car won’t look like this, after all.















“The highest efficiency thatcan be expected for a 5104M solution is 0.02%, and for a 2104M solution is 0.01%”
jesus that’s concentrated, what solvent? and how does it compare to current methods. cis-azobenzene (with the janky side-group) is very strained, so even other compounds might not compare, since it’s quite stable even in the strained state. perhaps other functional groups, at 4 and 4′ would be better?
No. It is not concentrated, it is a typo. :-) The paper mentiones concentrations of 2* 10^-4 to 5*10-4 M concentrations. The solvent is toluene.
*This*
One thing that really bugs me with media reporting on any technology/research related to energy is the propensity to jump to the grand claims that it’s going to be the next big breakthrough that will solve climate change or become the cornerstone of green energy. All to often, those claims are touted without any basis, and seem to exist solely for the purpose of making the topic at hand seem significant.
When it turns out that those claims are false, as here, (more below), it really damages the reputation of green energy as a concept. It hypes it up so much that enivitably, it has to pop, and when it does so, it makes it all look like a forced policy that exists only because of subsidies, politics, or whatever other reason people have to discredit green energy.
On the other hand, there is a real potential that this hype could actually result in technologies being adopted that don’t actually have solid basis in tech/science. And then when people try to critique those technologies, they get lumped in with people who “dont care about the environment” or “deny climate change” or “hate green energy” and so on.
All to say that hyping green energy tech has extremely harmful results on our society’s ability to agree to adopt efficient green energy (yes, energy production needs to be both green AND effecient). For an example of this, look back at the comments under the recient article on here about what should be done with wind turbine blades. The comments devolved into a polarized discussion arround, guess what, whether wind power was efficient enough to warrant its use, or whether it’s just used because of the hype.
It seems counterintuitive, but if you actually want to promote the adoption of green energy, and not just harvest clicks off of what many people see as a big issue, then you should REALISTICALY portray the science behind the issue. If reading the conclusion of a 7 page research paper to make sure your writing is accurate is too much to ask…. then you shouldn’t be writing about this stuff.
As for whether this stuff is a valid energy storage, well, the efficiency stats say it all. I encourage anyone who wants to know more to first go read the conclusion of the paper linked in the article (it’s half a page. Just do it). Then consider that solar panels have an effeciency of up to arround 23%. Compared to 0.01%. That is a 1000 times difference.
To make it even worse, this material releases its stored energy as heat, so we would need an entire additional energy conversion stage to make use of it. So that’s even more effeciency than drop. This makes less sense than a solar-powered fighter jet….
clap clap clap
Thank you
^this
^that
Finally, thank you.
So what your saying is that the best way to store solar energy for use at a later time is still a tree?
That would make an interesting comparison… energy storage in a battery, or energy storage in wood.
I mean said they thought biofuels were a good idea; go easy on ’em.
All these technological solutions for climate change are so underwhelming it’s almost hilarious if it weren’t so sad. We can keep fiddling with this stuff and hoping for a miracle, but in the mean time we gotta dismantle some carbon industry. Sorry rich people.
Azobenzene:
H302: Harmful if swallowed [Warning Acute toxicity, oral]
H332: Harmful if inhaled [Warning Acute toxicity, inhalation]
H341: Suspected of causing genetic defects [Warning Germ cell mutagenicity]
H350: May cause cancer [Danger Carcinogenicity]
H373 **: Causes damage to organs through prolonged or repeated exposure [Warning Specific target organ toxicity, repeated exposure]
H400: Very toxic to aquatic life [Warning Hazardous to the aquatic environment, acute hazard]
H410: Very toxic to aquatic life with long lasting effects [Warning Hazardous to the aquatic environment, long-term hazard]
That description of negatives effects sounds almost exactly the same as benzene. And trace amounts of benzene are found in all petroleum products (even some plastics). It is also added to gasoline to increase the octane. And benzene was also used to for lubrication in all pocket watches (which are extremely uncommon now).
Good grief! Calm down.
Energy storage lifetime of 40 hours.
Super-low efficiency.
Just an academic exercise – nothing real-world here.
Unfortunately true. Perhaps there is potential for improvement in this method.
Because storage is the true showstopper for renewable energy. PV panels are quite cheap already, batteries are not. If storage is not solved, the climate hysterics can forget most of their wishes for change in our energy system. Especially if they are against nuclear energy.
Real science is about incremental improvement in understanding, if you look hard enough most of what we think of as quantum breakthroughs were actually the culmination of countless years of research, hence why many “breakthroughs” happen at the same time by multiple people, e.g. powered flight (Wright Bros and Alberto Santos-Dumont) and Calculus (Leibniz and Newton) to name a few.
Research papers are more “Hey, this is interesting… maybe someone can improve on it”, and less “this will change the world!” I only wish reporting could/would reflect that.
Solar energy playing a vital role in developing countries and people are getting cheap electricity through solar panels. In the world lot of ways are introduced by people to get cheap electricity for short term and long term periods of time. All things are possible through research and reading of facts. We are introducing a library management system to find latest methods.
Chill Nathan,
Not talking about anything but “possibilites” from what I read. You might have to switch to decaf bro. Easy on take off Maverick.
About the same list is valid for regular gasoline:
Einstufung gemäß Verordnung (EG) Nr. 1272/2008
Karz.Kat.1B; H350 Kann Krebs erzeugen. Can cause cancer
Muta.Kat.1B; H340 Kann genetische Defekte verursachen. Can cause genetic defects
Asp.Tox 1; H304 Kann bei Verschlucken und Eindringen in die Atemwege tödlich sein. Can be deadly by swallowing and inhalaltion
Derm. Irit. 2; H315 Verursacht Hautreizungen. Skin irritation
Entzündbare Fl. 1; H224 Flüssigkeit und Dampf extrem entzündbar. extremely flammable
STOT 3; H336 Kann Schläfrigkeit und Benommenheit verursachen.
Repr. 2; H361fd Kann vermutlich die Fruchtbarkeit beeinträchtigen oder das Kind im
Mutterleib schädigen.
Aqua. chron. 2; H411 Giftig für Wasserorganismen, mit langfristiger Wirkung. Toxic for aquatic life with long term effects.
…and most of us use this stuff anyway.
I’d rather have a steam engine on my roof. Well, I think that’d be kinda fun anyway, but the neighbors might not agree….
Yeah, yeah. So continue drinking your benzene, won’t ya?
Not sure how residential hot water is managed elsewhere but in the UK it is quite common to have insulated hot water tanks and these have electrical immersion heaters intended as backup in case of gas boiler failure. I divert surplus power from my PV array first to my EV then to the immersion heater.
The trend now however is towards not having hot water tanks but to use on-demand heating of mains water. I will still retain the tank though so I can use it as a thermal store from surplus PV power.
Another method of thermal storage I cam across recently is phase change heat batteries, eg from Sunamp in Scotland.
Snake oil anyone?
How does this compare to the storage of heat in a super-saturated solution of water and sodium acetate, as used in some reusable hand-warmers? Has anyone experimented with using that as solar heat storage? The phase change during the (re)crystallization step might present problems, but likely not insurmountable ones.
Badly I think, iirc storage in lye was like 90% efficient (not easy to recover, but the heat was there).
Wow that’s one shitty paper to be referenced here.
yeh yeh nah, nasty stuff and all the meth heads would be stealing it as a feedstock for their drug labs.
What we need is a gigantic sodium acetate reusable hand warmer that surrounds your entire house that is charged up by a gigantic parabolic mirror. On a cold night all you have to do is back your car into a wall, and Vola, heat. I wonder what the efficiency of those hand warmers is. I would guess not much worse than .01%