To be honest, we originally considered throwing [Zachary Tong]’s experiments with ultralight metallic microlattices into the “Fail of the Week” bucket. But after watching the video below for a second time, it’s just not fair to call this a fail, so maybe we’ll come up with a new category — “Qualified Success of the Week”, perhaps?
[Zachary]’s foray into the strange world of microlattices began when he happened upon a 2011 paper on the subject in Science. By using a special photocurable resin, the researchers were able to use light shining through a mask with fine holes to create a plastic lattice, which was then plated with nickel using the electroless process, similar to the first half of the electroless nickel immersion gold (ENIG) process used for PCBs. After removing the resin with a concentrated base solution, the resulting microlattice is strong, stiff, and incredibly light.
Lacking access to the advanced materials and methods originally used, [Zachary] did the best he could with what he had. An SLA printer with off-the-shelf resin was used to print the skeleton using the same algorithms used in the original paper. Those actually turned out pretty decent, but rather than electroless plating, he had to go with standard electroplating after a coat of graphite paint. The plated skeletons looked great — until he tried to dissolve the resin. When chemical approaches failed, into the oven went the plated prints. Sadly, it turns out that the polymers in the resin expand when heated, which blew the plating apart. A skeleton in PLA printed on an FDM printer fared little better; when heated to drive out the plastic, it became clear that the tortuous interior of the lattice didn’t plate very well.
From aerogels to graphene, we love these DIY explorations of new and exotic materials, so hats off to [Zachary] for giving it a try in the first place.

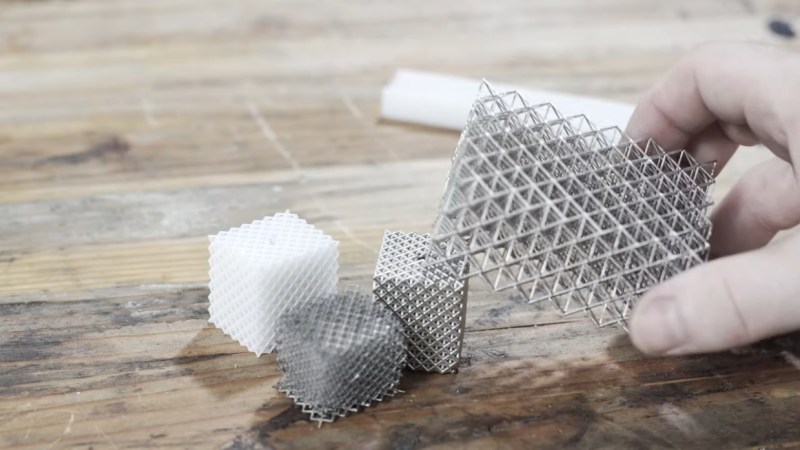











Definitely a fail, but those are the best for learning. Electroless plating is better option Caswell has an affordable nickel system solution based on cadmium not palladium.
Would pva disolved in water or hips disolved in limonene be worth trying?
I can remove excess from my hips with limonene?
Toss out the treadmill!
B^)
Worth trying for some purpose, but the scale they’re doing here requires SLA printing so they’re stuck with photo resin of some sort.
One could also try the opposite model, with a 3d print that has holes through it. A bit harder to get the paint and metal plating in, but it could be easier to get the plastic off. It could also work with a plastic cube that has grid of holes drilled into it.
Use quick set jelly and print with small drips.
Use warm water to disove it again.
You could make the jelly conductive.
Perhaps you could feeze jelly as you print it, then electroplate it.
vacuum sputtered titanium might be another easy method to do at home. Doesn’t need really really high vacuum to work, so a cheap pump and chamber is possible. That said I expect you won’t get good even coating – electroless does seem best to me at least as a first step.
Very neat idea, I wonder how much you can tune the lattice mechanically – to make some areas more crumply/stiffer/flex in only one direction easily etc.. With the SLA printer you can tune the form shape easily but actually getting even metal coatings over such complex geometry seems like it might add enough variation to rather badly effect the end result. Though I guess plating it really thick by design so the inconsistencies make up a smaller percentage could help as could some forced flow in the plating bath/ model of the plating process to tweak the lattice such that the more heavily plated parts are initially less dense by the right amount.
I think sputtering would have much the same problem, you get heavy coating on the outer parts of an object and very little in the middle.
If however, you have a higher resistance coating and clip to the middle of the structure with a pomona grabber or similar, then electro plating may build from that point outward, rather from point nearest opposite electrode.
Agreed on sputtering – just another option that might work and can be done at home easily. Really don’t think anything but electroless makes sense myself, but always worth trying might get a useful surprise – if you thread enough sputtering sources through the lattice you can probably get pretty even coverage but there’s always going to be occlusion (Titanium is if I recall correctly one of a few metals that don’t need the magnetic constriction of a sputtergun to sputter – just a good enough vacuum and some energy makes a whole rod shed).
The plating idea could work – might be best to have a little cluster connections inside so the whole mesh is relatively evenly charged too. Add in the agitation so the solution won’t get overly trapped and exhausted inside the lattice too (unless you are plating really really slowly so diffusion will keep up – which might also help get more even plating).
A better way might be to use a material that reacts with gas or liquid.
Perhaps you could print in conductive low melting temperature wax or even something like lard or butter, you could print it in a chest freezer and then plate that. Some experiments might be in order as to how conductive wax could be perhaps with carbon and fine metal dust. Lower temperature to drain the support materials would make for less oxidisation of the metal and allow you to plate thinner.
Maybe it could even be possible to make a metal foam if a fine mixture could be separated within a hot inert[ish] atmosphere.
There are conductive materials for FDM, but not so much for SLA.
A dual-head printer may make this approach viable, in combination with a dual-solvent removal of external, then internal, support.
I’ve done electroless nickel plating of SLA prints at home with relative success and ease. I wrote up the process on the Formlabs forum:
https://forum.formlabs.com/t/nickel-plated-prints/25459/10
Find out what it is that is used in spray on chrome processes to sensitize surfaces so that the silver will electroless plate out from the solution used. I suspect it’s tin chloride or stannous chloride. When glass is wet with either, then wet with silver nitrate, the silver plates out of solution and sticks to the glass.
If that process will work on plastics it should make a conductive coating that can be electroplated with other metals. There’s an order to electroplating metals, which ones are easier to plate over others. For chrome plating the best way is layer with zinc, copper, nickel, chrome. The zinc plates easiest and sticks best to steel, followed by the other metals in turn. The later metals in the order can be plated directly onto steel but will be more difficult and will tend to not stick as well. A lower cost chrome job that leaves out one or more of the other layers won’t last as long. That’s why in the same environment it’s typical to see better chrome on cars from the 1950’s and earlier (except during a couple of years during the Korean war when zinc and copper were rationed so many trim parts were chrome directly plated over lead or steel) than on cars from the 60’s and 70’s.
Where does silver fit in such a list? Dunno how easy or difficult it is to plate over or what it plates onto best. I’ll leave that research up to you.
Another item to consider is how well a plating back will “throw”, if one anode electrode on one side will drive the metal ions from the bath to the object from all directions (IIRC Gold does that) or if multiple anodes are required surrounding the object (as with chromium) because the metal ions will only move from the bath to the object in the space directly between the anodes and object.
Most metals can be bath plated with quite small amounts of power. Chromium requires one amp per square inch of object surface. Plating an old car bumper in a bath can take 1,000 amps or more. An alternative is “brush plating”. That uses a carbon or metal anode with a chemical resistant, absorbent covering. Dip in the plating solution and rub onto the object, just like painting. Adhesive masks can be used to create shapes in different metals and/or different amounts of them.
The mating surfaces on the connecting nodes of the ISS were brush plated because there was no way the manufacturer could dunk the whole node into a chrome plating bath and run the output of a large power plant through it.
I can verify Silver plating does work on DLP and (somewhat less well) on FDM (because of all the layer lines) using the stannous chloride sensitizing and Mirror making type reducing silver bath (kits online).
https://www.flickr.com/photos/andrewsharmon/27604562664/in/dateposted-public/