Lithium (from Greek lithos or stone) is a silvery-white alkali metal that is the lightest solid element. Just one atomic step up from Helium, this magic metal seems to be in everything these days. In addition to forming the backbone of many kinds of batteries, it also is used in lubricants, mood-stabilizing drugs, and serves as an important additive in iron, steel, and aluminum production. Increasingly, the world is looking to store more and more power as phones, solar grids, and electric cars continue to rise in popularity, each equipped with lithium-based batteries. This translates to an ever-growing need for more lithium. So far production has struggled to keep pace with demand. This leads to the question, do we have enough lithium for everyone?
It takes around 138 lbs (63 kg) of 99.5% pure lithium to make a 70 kWh Tesla Model S battery pack. In 2016, OICA estimated that the world had 1.3 billion cars in use. If we replace every car with an electric version, we would need 179 billion pounds or 89.5 million tons (81 million tonnes) of lithium. That’s just the cars. That doesn’t include smartphones, laptops, home power systems, massive grid storage projects, and thousands of other products that use lithium batteries.
In 2019 the US Geological Survey estimated the world reserves of identified lithium was 17 million tonnes. Including the unidentified, the estimated total worldwide lithium was 62 million tonnes. While neither of these estimates is at that 89 million ton mark, why is there such a large gap between the identified and estimated total? And given the general rule of thumb that the lighter a nucleus is, the more abundant the element is, shouldn’t there be more lithium reserves? After all, the US Geological Survey estimates there are around 2.1 billion tonnes of identified copper and an additional 3.5 billion tonnes that have yet to be discovered. Why is there a factor of 100x separating these two elements?
What is Lithium and Where Does It Come From?
Lithium is geologically rare because it is unstable atomically due to it having the lowest binding energies per nucleon than any other stable nuclide. This is good for nuclear reactions (lithium was used as fuel in the first early nuclear reactions in 1932) but bad for finding it in nature. Further compounding its volatility, lithium is an alkali and will combust if allowed to come in contact with elements it reacts with, such as those found in the air. Pure lithium needs to be stored in oil to be transported safely.

Given that it’s rare and reactive, the process of extraction differs from other metals. Currently, there are two ways that lithium can be extracted. The first way is from ionic compounds, such as pegmatitic minerals (made of quartz, feldspar, mica, and other crystals). For a long time, this was the world’s primary source of lithium. Much of Australia’s lithium production as of 2020 comes from spodumene, a pyroxene mineral that occurs in pegmatites and aplites.
In addition to being in minerals, lithium can be found in brines and ocean water because of its solubility as an ion. This means lithium saturated brines found in South America and Nevada can be dried using a solar evaporator, then once a good concentration is reached, the lithium carbonate and lithium hydroxide are precipitated by adding sodium carbonate (washing soda or soda ash) and calcium hydroxide (slaked lime or caustic lime). The brine extraction process usually takes 18 to 24 months.
However, the different processes are not equal in the lithium they produce. As mentioned earlier, battery manufacturers require 99.5% pure lithium and the remaining 0.5% is important. Brining tends to bring in iron or magnesium, impurities battery manufacturers try to avoid. Spodumene also has the advantage of purity as the deposit in Australia is an estimated 2.4% lithium. However, the sheer abundance of lithium in brines makes it very compelling even though the concentration is much lower (0.2-0.3%). An estimated 230 billion tons of lithium is dissolved in the oceans, but at 0.1-0.2ppm, it will be a while before extraction becomes economically viable. The difference between the identified reserved and the total estimated reserves can be mostly explained by brines. Brines are hard to estimate as they vary in concentration drastically and are often hidden in strange locations.

For example, most of the world’s lithium brines are concentrated in a region known as “The Lithium Triangle”, an intersection of Chile, Bolivia, and Argentina. This triangle is believed to contain over 75% of the existing known lithium. One such source of brine is the Salar de Uyuni salt flats in southwest Bolivia near the top of the Andes (almost 12000 ft or 3700 meters above sea level).
There’s a layer of salt on top that ranges from a few centimeters to several meters thick. Underneath the hard crust is a liquid brine with a relatively high concentration of lithium (0.3%). A hole drilled into the crust allows the brine to be pumped out and processed. As you can imagine, the high altitude complicates extraction and makes it more complex to transport the extracted lithium.
Battery Technologies
Why does lithium work so well as a battery? The myriad number of battery varieties using lithium seem endless. There is Li-MnO2, the most common consumer-grade battery chemistry, Li-FePO4, Li-CSVO, Li-CFx, Li-CuFeS, and Li-FeS2 are just some of the variants that are in common use today. Lewin Day wrote a beginner’s guide to lithium batteries that can help sort them out.
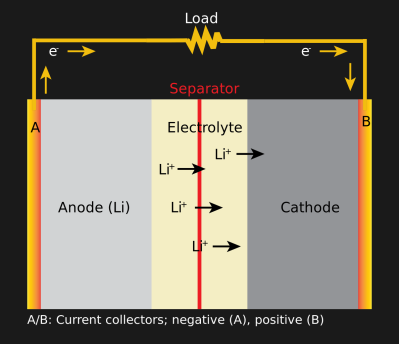
Every battery has three parts: an anode, a cathode, and an electrolyte. Most batteries today use a liquid electrolyte, which is made of lithium salts suspended in an organic solvent. Solid-state lithium batteries offer some promise but are still very much in development. They use solid lithium oxides as their electrolytes because being solid, they cannot leak, which is a safety issue for their liquid-based counterparts. The anode is often a material such as graphite or lithium titanate intercalated (a reversible layering) with lithium. Cathodes are often made from lithium nickel cobalt (LMO) or Lithium nickel manganese cobalt oxide (NMC).
The reversible reaction inside a lithium battery is quite similar across all lithium batteries. During discharge, an oxidation half-reaction occurs as the anode that forms negative electrons and positive lithium ions. The lithium ions head towards the cathode while the electrons move through the circuit towards the anode. They recombine there in a reduction half-reaction. Apply an electrical current and this reaction is reversed. Since both the cathode and the anode allow the lithium ions to intercalate within their structures, the lithium ions are said to “rock” back and forth between the two electrodes. Thanks to lithium’s relative instability and atomic structure, it is easy to form a lithium-ion and transport it through the battery.
This reaction does have its limits. Overvolting a battery (5.2 volts) leads to the synthesis of cobalt oxide, which causes damage to the electrodes. Letting the voltage potential drop too low results in the production of lithium oxide, which damages the battery irreversibly by reacting to the battery itself. You can learn a bit more about battery chemistry in this wonderful Bob Baddeley wrote last year.
Cathode/Cobalt issues
As I mentioned, the cathodes of batteries are often made with cobalt. Funny enough, cobalt is in someways rarer than lithium. Despite its rarity, the demand for cobalt has continued to skyrocket thanks to lithium-ion batteries. More than half of the world’s supply is in DR Congo, which is an infamously exploited area of the world. Child and slave labor are repeatedly reported in the mines and many companies have tried to find ethical sources of the material. Progress has been made to reduce the amount of cobalt required per battery and it has dropped from 1/3rd (NMC111) to 1/10th (NMC811) of the cathode. Many companies are trying to make batteries without cobalt entirely, for example, the Tesla Model 3 has an LFP cathode which is cobalt-free. Even with new technologies being put into production, we are still a long way from being cobalt-free and the majority of batteries today are still cobalt-based. We may face a cobalt shortage long before we face a lithium shortage.
Do We Have Enough For Everyone?
The short answer is probably. Dozens of different Universities and National Labs have come out with studies predicting one way or another. Lawrence Berkeley National Lab said in a 2011 study that we could build a billion 40 kWh lithium batteries with our existing reserves, however, they assumed only 10kg of lithium per battery (1/6th of a Tesla Model S). Even if we have enough raw materials, the process of converting it into a usable form needs to be considered.
Consumption has grown around 25% per year since 2012, outpacing the 4 to 5% yearly gain in production. At some point, something is going to have to change. Several have compared these market conditions to the oil industry. The demand for oil led to new methods for extraction and new technology that weren’t commercially viable before. We still have a large amount of research and development to do before extracting lithium from less concentrated sources such as the ocean becomes more economically viable. That said, there are a few things we could do that might ease the bumps along the way.
Recycling Lithium
Recycling lithium has been a dream of researchers and engineers alike. A few hurdles stand in the way of that dream, namely designing for recycling and cost-effectiveness. Unlike lead-acid batteries, which are designed with recycling in mind and achieve around a 98% recycling rate by mass, lithium-ion batteries are often focused on fitting the size and shape of the product they are in. Recycling also requires labeling to tell what chemistry the battery is, which lithium-ion batteries often do not. Unlike lead-acid, lithium batteries have anodes and cathodes of similar density, which makes them hard to separate out for recycling. This requires complex chemical or magnetic separation steps that vary according to the battery chemistry.
 That said, universities are working to improve the process. While their results are incredibly promising, there’s still the problem that recycling is not economically viable yet. Just buying the raw materials and making new batteries is cheaper than recycling old ones.
That said, universities are working to improve the process. While their results are incredibly promising, there’s still the problem that recycling is not economically viable yet. Just buying the raw materials and making new batteries is cheaper than recycling old ones.
New Battery Technologies
Every few years or so, some new battery technology gets heralded as our potential savior. It seems that the promises of new and better batteries being just around the corner are held up each year anew. We’ve covered the current contenders as well as some promising upstarts such as lithium-sulfur and lithium-ceramic. Each promises different things like higher energy density, faster recharge, or being more environmentally friendly. While the general consensus is that we’ll believe the battery breakthrough hype when we see it in a consumer product, we still must give credit to all the researchers and engineers over the last few decades creating the steady stream of improvements to the lithium-ion battery.
So next time you look at the small lithium battery in your project or the large bank of 18650 cells, take a moment to appreciate where they came from and perhaps even allow yourself to wonder what will come next.
















“due to it having the lowest binding energies per nucleon than any other stable nuclide.”
Measure twice, cut once. “lowest…than” needs editing.
“lithium was used as fuel” Harrumph. “Target” not “fuel”. Might as well say film is fuel when you take an x-ray.
Lowest… among all of the stable nuclides
I was going to say, lithium as fusion material wasn’t discovered until (long?) after they split uranium. Also see the Castle Bravo test, where the bomb had a fusion yield MUCH higher than predicted because they hadn’t checked out both of the isotopes!
Lithium is the 25th most common element on Earth https://en.wikipedia.org/wiki/Lithium
Although it was synthesized in the Big Bang, lithium (together with beryllium and boron) is markedly less abundant in the universe than other elements. This is a result of the comparatively low stellar temperatures necessary to destroy lithium, along with a lack of common processes to produce it.
It doesn’t matter (just yet) how common lithium is in the universe at large. The stars are beyond us, even the earth’s core and mantle are out of reach, only the crust matters. Atom for atom lithium is more common in the earth’s crust than chlorine (or copper, nickel, cobalt and lead) (see link below). Every winter we splurge millions of tons of chloride ions onto our icy roads and no one is talking about a chlorine shortage. The only reason lithium is “rare” is that we haven’t needed any more than we had found, so we stopped looking. As the article mentioned the same happened with oil. Even helium, it was thought the only source was natural gas because the biproduct met our demands. When there was a little worry about that, it didn’t take long for geologists to find a helium vent in Tanzania.
https://en.wikipedia.org/wiki/Abundance_of_elements_in_Earth%27s_crust#/media/File:Elemental_abundances.svg
> Pure lithium needs to be stored in oil or sealed away from reactive elements like air.
Last time I checked air wasn’t an element.
If fire, earth and water can be elements…
lol; Earth, Air, Fire, Water elements
Then, a hundred years ago, the lithium nation attacked.
But when they met the water nation, they accidentally founded the fire nation.
They meant it in the sense of “things”. Like saying there is an element of suprise.
Everyone already knows air is not an element and read it that way except you, apparently.
The scientist who discovered the element of surprise was not expecting the results he obtained.
Yeah, not being a native speaker often makes me cringe at things nobody else even notices.
“[S]ealed away from reactive elements like oxygen” would go so much more smoothly, though.
If we could get people to quit smoking it…
“high altitude complicates extraction and makes it more complex to transport”
? Lower boiling points for drying stuff out and downhill to all markets.
Suggest making large lithium disks and then something like a cheese rolling competition to get them down the mountain.
Given how reactive it is, that would be rather like chasing some flaming saganaki downhill. Opa!
> It takes around 138 lbs (63 kg) of 99.5% pure lithium to make a 70 kWh Tesla Model S battery pack.
That’s WAY too high an estimate. U of M estimates 11.6 kg for an 80 kWh NCA battery: https://energy.umich.edu/te3/wp-content/uploads/sites/2/2018/11/Evan-Leon.pdf
You can do some basic arithmetic to see how wrong 63 kg is. 70 kWh = 5750 to 5850 NCR18650B cells. The 85 kWh Tesla packs have 7,104 18650s, which scales to 5,850 for a 70 kWh pack. Each 18650 weighs 47.5 grams, +/- a couple grams (battery manufacture is surprisingly variable). Total cell weight is 273 to 278 kg.
Those cells would be 23% lithium by weight. That’s so, so wrong. An example NCA chemistry would be LiNi(0.84)Co(0,12)Al(0,04)O(2) which is 7% lithium by mass. Even if you ignore the entire anode, separator, electrodes and case, and assume the entire cell is made of cathode material, the total amount of lithium would be 19.8 kg. Typically the cathode makes up closer to 50% of the weight, and there’s a little bit of lithium in the electrolyte. For current chemistries the amount of lithium is <3% by weight.
The author probably mixed up lithium and lithium carbonate.
I suspect the problem of Lithium supply will be solved shortly. There’s quite a bit of Lithium in sea water, and some promising technologies to extract it.
The most recent I read about talked about electrolytic extraction – essentially plating an electrode with lithium. You start with a plating setup using sea water as electrolyte and use a membrane barrier to prevent most of the ions from reaching the plated electrode. The ions that get through are sodium and lithium.
The sodium was thought to be a problem, but the new process periodically reverses the electric potential. Sodium is faster than lithium (if I’m remembering that right), so the sodium ions come off and go back through the membrane faster than the lithium ones. Cycling the plating process can tune the plated material to be mostly lithium.
Another technique used zeolites. Informally, zeolites have molecular holes that can capture ions from solution selectively by size and valence. People have made specific zeolites to capture thorium and dragged long “socks” full of the stuff through sea water to make a zeolite/thorium mixture that was equivalent to thorium ore. Thorium is very common so this is not a good fit for thorium mining, but it might prove useful for lithium. (I suspect the researchers chose thorium to get a grant to study nuclear resources.)
Fritz Haber felt that gold could be mined from sea water, and ultimately found that the concentration would need to be about 3x higher to make the process economically viable. For a back-of-the-envelope calculation, take the concentrations of gold and lithium in sea water and compare to their current spot prices.
I’m generally not an overly environmentally conscious person. But in terms using a source of water to be treated in order to harvest a particular chemical or chemical compound. To me, seems like we need to look at the environmental impact before we decide to start investing in pumping billions of gallons of water just to harvest a currently an non renewable and non recyclable resource that is also reactive.
My biggest concerns would be:
What kind of processing by-product is produced?
For example fracking basically pumps the detergents and waste byproducts back into the hole the oil was pumped out of. If that chunk of earth fractures while the tectonic plate is moving around the waste products could contaminate a food or water supply for an area.
What kind of chemical chemical properties will processed waste water have?
After the sea water has been processed, if there is still “water” that is left and it isn’t packed full of harmful chemicals and an environmental agency or even a shady business decides to dump the waste water back in the ocean. How will that affect the PH level of the ocean? Will the returned water have other chemicals stripped from it?
The last issue is, the worlds drinkable water is diminishing. It may still be a few hundred years away, and the alternate source would be to filter the ocean water. What would pumping billions of water just to keep up with our demand for energy do to the timeline before we would run out usable water? As in Water that can be processed to drink?
As a final thought, I think everything needs to start having a reasonable level of research into how can this new product:
Be made with more abundant resources and more re-usability before it needs to be trashed or recycled.
There needs to be a feasible recycling process available or in the works.
I understand this a tall task, but at some point we are going to have a convergence of things that require the processing of one resource that will impact and render other resources useless. And all this could happen before we even find a new place to live or new source for the materials.
You’re not going to pump billions upon billions of gallons of ocean water, that would be an incredibly massive waste of energy. Extraction techniques use large scale “kelp farm” style structures, where the membranes or zeolites or adsorption materials are strung out in ocean currents, left for a while, and then retrieved for processing. Some of these might get lost or break down, but the ocean is incredibly huge and in the scale of things losing a few or even comparatively a lot of these would have essentially 0 impact on the ocean water.
The ocean is big – really big. There are approximately 362 quintillion or 362000000000 billion gallons of water in the ocean. Taking a billion gallons of water out of the ocean would be comparable to taking a few hundred or thousand gallons out of one of the Great Lakes.
And if that doesn’t work out, Chile, Bolivia and Argentina are the next lucky recipients of freedom.
Don’t forget Mexico, #1 top ten
https://www.mining-technology.com/features/top-ten-biggest-lithium-mines/
Freedom as in Evo Morales (ex-president of Bolivia) having to go into exile?
due the cue in wich the USA was involved?
don’t worry, the other nations are cueing up to start a coup if the US doesn’t; specifically France, Great Britain, Germany, Russia, and newcomer China.
The US tends to act as cleanup crew for the first three whenever stuff goes awry.
If lithium is a problem, why not go back to lead-acid batteries and just use less electricity?
Because energy density of lead acid in comparison to lithium batteries makes their use impractical for a number of applications. Also who in their right mind wants to go back to lead acid batteries in portable electronic devices?
The only thing I can think of that would be impractical is anything flying; drones, etc. We had electric cars that ran on lead-acid batteries over 100 years ago, and they worked fine. As for portable devices, maybe people wouldn’t be glued to their phones as much if they were heavier. I don’t see a problem there.
Lead acid batteries are easy to make, easy to recycle, and add value to a common metal that doesn’t get much use otherwise. Underrated tech IMO.
“The only thing I can think of that would be impractical is anything flying;”
I don’t think we want everyone driving 10 ton cars around. Enough people die in car accidents already.
Why would they be 10 tons? Where are you getting that from?
Basic math: take approximately 50 kWh worth of batteries for a reasonable amount, at 35 Wh/kg, and you get 1,429 kg of batteries. Then times that by 5 because you don’t want to hurt your batteries by deep-discharging them. That’s 7 tons of batteries.
To move it, you need a vehicle that is rated to carry the amount. That would be a Class 4 medium truck, such as a Ford F-450 which by itself weighs about 3 tons dry, give or take, which gets you a 10 ton car.
Only, it’s not that simple because you can only load up to about 3 tons on the car itself, so you’d have to tow the rest of the batteries behind in a trailer. That’s just to get the same amount of usable energy as in a Tesla Model 3.
Of course you could just leave it at 1.4 tons of batteries and make do with a smaller truck, but then it’s possible you can kill the battery in less than 50 charges by going the full distance.
@Dude
50KWh is absurd. When I ride my bicycle, my maximum continuous power output is roughly 200W. Assuming you’d want to travel for 10 hours straight, that’s 2KWh. The goal, then, should be to make a car that is as light and efficient as a bicycle, not scale up the power so we can keep driving enormous/wasteful cars.
Higher energy density just enables wasteful behavior. Learn to make do with less, and the quality of engineering will be forced to improve. For a software example, consider programming today vs. programming 30 years ago. Bloat exists because it’s allowed to exist, and this doesn’t just apply to computers.
We’re not talking about bicycles but cars. It is not a subsitute.
50 kWh is the chemical energy content, or the heat of combustion, of 1½ gallons of gasoline. In order to make a car go any distance with that energy, it already has to be extremely energy efficient, which a car that can carry 50 kWh worth of lead-acid batteries isn’t.
> consider programming today vs. programming 30 years ago
30 years ago you made bespoke software that was optimized for a single type of machine, using all the limited resources available there, which was often not enough. As a consequence, you had a very limited user base, and the cost of development and maintaining the software on a per-user basis was high. This meant that very few could even afford to have the software, it was unpolished and difficult to use, and it was used sparingly only where absolutely necessary, which meant it was of very limited scope and impact to the society at large. In other words, it was stuff that only weenies cared about.
Now you make software that has to work on all machines and be easy to develop, so it could have billions of users who can afford it at a very low price point, so it would be useful to the many and have a high impact to the society.
Your choice.
>Higher energy density just enables wasteful behavior.
Lead acid batteries are extremely wasteful, both in terms of material resources and their electrical efficiency.
(data from wikipedia)
Lithium Ion
Specific energy 100–265 W·h/kg
Energy density 250–693 W·h/L
Lead Acid
Specific energy 35–40 Wh/kg
Energy density 80–90 Wh/L
the volume density of lithium ion is anywhere from 3-7 times that of lead-acid. lets work backwards now:
a representative cell phone battery might be 5cm x 8cm x 0.5cm (20cc), for 2000mAh @3.7v (7400mWh), or 370 Wh/L, a reasonably middling performance
the equal capacity of (ideal, 90Wh/L) lead acid would be 88cc, or 2.2cm thick in the same footprint as the above Lithium pack. This is without getting into charge/discharge rates and voltage requirements of electronics, either
I don’t think that’s going to fit in a pocket.
So just have less capacity. Did you really not consider that option? Just *use less electricity*.
So instead of charging your phone once a day, you want to recharge it three times a day? or are you suggesting that in this world where companies are bragging about their long battery lives, they are intentionally ignoring ways to increase their battery lifespans by two thirds.
@Steel_9
I have a flip phone, I charge it once or twice a week. Just make phones simpler and lead-acid batteries would be fine.
Your flip-phone has a 900 mAh 3.7 Volt standard lithium battery. Approximately 4 Watt-hours in capacity. To achieve the same performance out of a PbA battery, you would need one that weighs 114 grams which is heavier than the entire phone is, but it gets worse because you could not use that capacity without destroying the battery. Assuming a 20% DoD to keep the battery happy for at least 500 recharges, it would weigh half a kilogram.
It wouldn’t be a flip-phone but a brick-phone.
Though we don’t have to guess what a lead-acid cellphone would look like, because it’s already been done:
https://upload.wikimedia.org/wikipedia/commons/0/06/Motorola_Bag_Phone_Outside_Bag.JPG
It has a 12 Volt 2.3 Ah battery, which has a technical capacity of 27.6 Wh but you can only use about 5-6 Wh without killing it in the long term. “The battery provides up to 2.5 hours of talk time and 48 hours of standby time.”
Then there’s laptops, which usually have 40-60 Watt-hour batteries. Nobody has ever tried to make a laptop that runs on PbA batteries, because you would have to add wheels to it and cart it around like luggage.
@Dude
People can figure out how to make things use less power. If a gameboy could run for a day on two AAs in the ’90s, we can figure out how to do the same with a phone or a laptop. Just scale down the display size and processing power.
> Just *use less electricity*.
To re-iterate, the useful specific energy of lead-acid batteries is actually only about 1/10th of a lithium battery, because deep discharging destroys the battery very quickly. Most of the battery powered applications are only practical because we have something better than lead batteries, because you just can’t use 90% less energy for the same effect.
> If a gameboy could
A gameboy would run 15 hours on 4xAA back in the day.
>Just scale down the display size and processing power.
And then not do what we built the thing to do. There’s a minimum useful size for a laptop screen for example, where you can no longer fit enough documents on the screen, or enough of a document to work on it.
Laptops have pretty much standardized around 14″ because while people would like to have a larger screen, any bigger would not fit in a bag comfortably and it would be too heavy, and any smaller couldn’t carry enough batteries to actually work for a day.
@Dude
I’m pretty sure we could make big monochrome LCDs that you could edit documents on, and they wouldn’t use much power.
Fundamentally though, at some point we need to tell people “no”. People will always want ridiculous things, and the free market does a great job of enabling them. We should probably stop that.
The documents I edit are in color, which is immensely useful for differentiating variables in a graph. I don’t want to go back to the 70’s for this. You’re just out of date.
@Dude
“You’re just out of date.”
No such thing. Anyone can use any technology from any time; there’s no expiration date on something that works. Consider the success of e-book readers if you think monochrome is unusable.
>No such thing. Anyone can use any technology from any time
Then I do suggest you try to use a spirit copier next time you need to deal with some paperwork in a modern paperless office.
>Consider the success of e-book readers if you think monochrome is unusable.
Which is not the same goalposts.
I find that nobody uses them for more than reading a book exactly because they lack in color reproduction and the update rate is painfully slow, so interaction with the software is made difficult.
@Anonymous: At some point we have to say “NO” to you and all the environmental hysterics. We (I and the majority of people) do not go back, (nearly) nobody want’s this.
We have smartphones now with fast CPUs and good high-res displays, we have cars now with powerful engines and we want AND WILL keep them. If necessary defend against environmental hysterics who want to question our current lifestyle.
A bicycle is primarily a sports device which only people from poor countries have to use and environmental hysterics want to use as a vehicle.
Yeah, we definitely need more SA and Pb , I would especially like it in my phone and my vape, I’m sure contamination wouldn’t be likely, and hell, have you ever heard of either hurting anyone?
A leaking lithium ion battery is much worse, and most of the fear surrounding lead is irrational. Don’t eat batteries, pretty simple.
A lead battery tossed in the environment leaks lead sulfate, which is considerably more water soluble than metallic lead or lead oxide, which makes it an immediate environmental hazard because it gets around by leaching, and it’s a contact poison that bio-accumulates in people and animals. In old scrapyards with piles of dismantled car batteries, even the dust you’re kicking up contains lead sulfite that is easily absorbed through the lungs.
A lithium battery doesn’t have such hazardous chemicals. The chemicals inside a li-ion battery may be hazardous if ingested in concentrated form, but they present no danger if they happen to leak into the environment in low concentrations.
@Dude
Lead sulfate is harmless lmao, they used to sell it as “non-toxic lead white” in paints. You’d have to eat a pound of it to get sick. Most of the scaremongering around lead comes from bad data from the 70s, where some scientists noticed that dumb kids ate paint chips. Correlation = causation, after all. I don’t buy it.
Yes, used to, until they figured out it’s not safe after all.
>You’d have to eat a pound of it to get sick.
If you ate a pound of it, you’d die.
>Correlation = causation, after all. I don’t buy it.
Lead poisoning starts to have an effect at 5 micrograms per deciliter of blood in children, 10 micrograms in adults, although you can tolerate much higher levels through gradual exposure because chronic exposure de-sensitizes you to a point. However after you’re at 40-50 micrograms per dl, you will start to show symptoms anyways. This is clinically proven.
For a 30 kg child, it’s enough that they absorb 12 milligrams of lead in some bio-available form, such as from lead sulfate. This will cause developmental defects such as lowered IQ. Eating paint chips and chewing on toys with lead paint on can indeed poison you.
Notice that inorganic lead, metallic lead, isn’t readily bio-available and things like the use of leaded solder (with good practices like not chewing on it, washing your hands after use) does not appear to have any impact on blood lead concentrations.
Lead exposure even in small amounts is bad because it isn’t eliminated by the body. It is stored in the tissue and bones, and is only slowly eliminated over 20-30 years once it gets into your bones. For acute exposure, the half-life is about 40 days (about a year to return completely back to normal), so you can see why children eating chips of lead paint can lead to a buildup very quickly even if it’s only minimally absorbed.
@ Dude
If you bothered to read my post, you wouldn’t have needed to recite all that. The “5 micrograms per deciliter” figure comes from a study with a small sample size that based their findings on outliers. Like I said, bad data. Prior to the 60s/70s there was little evidence that sub-acute lead poisoning has any effect on IQ, because it was standard practice to exclude children with pica (eating things that aren’t food) from the studies. Pica correlates with low IQ independently of blood lead levels, so including kids who eat paint chips in your lead studies is a confounding factor.
Please stop assuming that people who disagree with you are stupid/trolling/misinformed.
>Please stop assuming that people who disagree with you are stupid/trolling/misinformed.
Please stop trolling if you don’t want people to assume you are. Mr. “Lead sulfate is harmless lmao”. You give me very little options here.
>The “5 micrograms per deciliter” figure comes from a study …
It’s actually supported by many studies. The same results crop up everywhere they test: no amount of lead in your blood is “safe”, plus it’s quite easy to get way more than 5 micrograms because the amounts you have to ingest are quite small, and it accumulates over time, so there’s no grounds to support your assertion that lead sulfate, or discarded car batteries as it were, is harmless or negligible in any sense.
https://jamanetwork.com/journals/jama/fullarticle/2613157
As a summary, the article above does not concern with children who eat paint, but who had exposure due to severe air pollution in New Zealand. The levels range from 4 to 31 µg/dL, the sample size is 1007 individuals, and “each 5-µg/dL higher level of blood lead in childhood was associated with an additional 1.61-point lower score”. Another finding was that children under the level of 10 micrograms showed a slight increase from childhood to adult IQ, while children at or above this level showed a decline.
It’s practically pointless to debate whether 5 or 10 micrograms should be considered “safe” if you can avoid both by not tossing your old batteries in a scrap pile ’round the yard.
@Dude
Did you read the study you posted, or just the abstract?
Mean IQ at adulthood vs. childhood blood lead levels:
15ug/dl: 99
Wow, what an emergency this is. Definitely worth banning one of the world’s most useful metals over. Or maybe not; looks like a little bit of lead makes you smarter. Look for lead supplements in your local pharmacy!
Looks like something broke the formatting, let’s try again:
under 5: 101 IQ
5 to 10: 103 IQ (!!)
10 to 15: 99 IQ
over 15: 99 IQ
Not sure what SA is but lead batteries? Yes. Go to Google, type in “Lead batteries hurting someone”. According to battery university, top of the fold and a generally reliable source of battery technicals, “blindness and death”.
They’re full of acid and I can weld things with the power output.
Lead acid batteries are also plagued by issues that modern technology solves with ease.
The biggest issue with lead acid batteries is that they hate being deeply discharged. (A cell voltage bellow 1.85 volts is undesired.) Mainly due to a lead sulfide layer forming and when it is sufficiently thick it can start flaking off. A very similar process to how iron corrodes away…
And the fact that the vast majority of lead acid battery packs/installations are unbalanced is only worsening the issue…. (One can “balance” them by overcharging them, it only results in the electrolyte boiling off, primarily due to the water splitting into hydrogen and oxygen…)
But toss on some cell balancing onto lead acid packs and they can last a lot longer.
Not to mention that lead acid batteries loves being fully charged. Making them ideal candidates for UPS installations. (An application where their lower $/kWh compared to lithium is also another bonus, not to mention that weight isn’t a big issue. And in terms of size, lithium isn’t actually that much smaller… (Especially when looking at a whole UPS system.))
The main issue Lead acid batteries have is:
1. A long history of abuse and resultant failures setting a precedence that the batteries are unreliable.
2. A somewhat toxic composition. (As if cobalt is any safter…)
3. They are usually fairly heavy…
But for grid storage, lead acid should be the go to solution as far as batteries are concerned. Not lithium batteries that frankly hate being fully charged long term. (And lead acid cells have similar discharge losses, ie, power density isn’t a major difference.)
Not to mention that a properly maintained lead acid cell that hasn’t seen excessive discharges can last for decades. Only needing some more electrolyte added every 5-10 years.
Though, people have similar opinions about lead acid batteries as people have about compressed air as an energy storage medium. Obviously, since shop compressors are inefficient, mainly due to aiming at high flow, then compressing air is just inherently inefficient.
And lead acid batteries are just inherently bad due to usually being in a 6 cell series string without balancing and relying on overcharging to keep it moderately in check, while also considering 10 volts as the “empty” state, despite manufacturing tolerances between cells being above 10% typically… (ie, 1 cells is well bellow 1 volt, while the rest happily sit near 2 volt. The smallest cell eventually dies surprisingly enough… It is also the cell that boils of electrolyte the fastest if you have a non-“maintenance free” battery that is.)
Yes, lead acid batteries are an underrated technology that frankly is better than lithium in some applications.
Another technology that has poked my interest is capacitors.
Modern super caps I have looked at stores about 25-40 kJ/kg, not all that bad compared to lead acid (126 kJ/kg) or lithium (360-900 kJ/kg). And capacitors are steadily getting better, and they survive fairly decently, most are rated for 10+ years of continuous operation without greatly leaving specs, for a phone it could honestly be an interesting technology in itself, potentially for cars too, but the material requirements for these is another can of worms to explore….
Lots of good points here. I think a lot of people, even self-described “hackers”, tend to fall into the trap of letting industry lead the way. Technology that is the most profitable, or even the most efficient, isn’t necessarily the best technology in the long run. Simplicity, elegance, and longevity often take a backseat to more immediate economic rewards, and everyone else ends up stuck along for the ride as we cycle through “innovation” after “innovation”, when often there’s been a reliable solution in existence for decades or even centuries.
Yes, it’s great that Tesla uses X, or Samsung uses Y, but people need to look at the big picture too, not just the watts and volts in the here-and-now.
>But for grid storage, lead acid should be the go to solution
It would be tremendously expensive for what it can do. For the same problems as you mention, it only has around 10-20% actual capacity to avoid deep cycling, and it lasts only a few years before it must be replaced, and there’s a problem with scaling the system up. Simply calculate the tonnage of lead you’d need to store 1 TWh of energy, and how fast you’d need to be recycling it to keep up with demand.
Yet this wouldn’t be nearly enough. US electricity consumption over an average day is ~11 TWh. For any grid storage solution, for strategic reserve (1-3 months) and seasonal storage of renewable power, 100 TWh would be a good starting point.
Let’s also not forget that the lifecycle ESOEI of a lead-acid battery is about 2, where for lithium batteries it’s around 10. That translates to efficiency: if you manage to fully utilize the maximum storage potential (optimal number of discharges per day per capacity), your energy efficiency will be about 50%, which is terrible.
Even if assuming the batteries cost nothing, the low life cycle efficiency means doubling the cost of the energy stored with the batteries because you’re effectively using twice as much, so if you’re thinking in terms of renewable energy + batteries, the result comes out as “too expensive for the grid”. In reality it would be worse because the recycling infrastructure wouldn’t be using the more expensive energy, but something cheaper like natural gas or coal to make it economically viable in the first place.
Some of us like to get more than 20 miles away from the city before running out of lead acid.
Since they’ve banded lead in solder, fishing sinkers and who know what else, there is most likely a surplus of it
Nearly all the lead we produce goes into making car batteries in the first place. The rest is rather niche.
No, electric cars with lead acid did not work “fine”. They worked – somehow – but in no way comparable with todays fuel powered cars. And that is – for me – the comparison, the goal in performance, price and range.
“just use less electricity” … sure also let’s just ignore laws of physics and handwave away any reasonably valid concerns about the limitations of lead acid in order to force it into applications where it is unsuitable
Have you ever considered that it isn’t the battery which is unsuitable, but the application instead?
Typical engineer mindset to never ask “why?”.
Wow, that’s the stupidest circular logic I’ve heard in awhile. Dressing up emptiness to make it sound poetic doesn’t produce substance. Putting other’s down for your own social shortcomings doesn’t make you look intelligent, just petty. Cant win an argument like an adult, so resorts to childishly demeaning an entire group.
There’s nothing circular about it. Given any problem, you’re always free to reject the premise. Engineers never do this, which is why they’re such reliable employees. Looks like I touched a nerve by pointing that out.
No, you’re just trolling.
There wouldn’t be smartphones with lead acid batteries, because they wouldn’t fit your pocket. Your argument boils down to “stop using everything that uses lithium batteries – problem solved”, which isn’t a solution or an alternative but simply accepting failure and calling it something else.
It’s the same as re-branding rolling blackouts and energy rationing to be “demand management”, or calling the murder of civilians in a warzone “collateral damage”.
>Engineers never do this
Because when given a problem along the lines of “solve the world’s hunger”, they assume the point is to benefit the people who are living in the real world, not playing armchair philosophers by saying “kill half the people and feed them to the other half”.
@Dude
Accepting “failure” is often better than coming up with solutions. For example, you can’t solve world hunger; the more you feed, the more they breed, the more they need. Starvation actually works as an alternative because it keeps the population in check.
But you just presented the solution: keep the population in check.
The only question then is, whether you do it by letting people starve to death, or something more humane perhaps?
You see, there is always a solution. Something will happen anyways, so you can’t get out of the responsibility of the choice by “rejecting the premise” that there is anything to solve.
By choosing not to offer solutions, you are offering the default solution which is to let people die in misery, which you can already predict to happen. It’s like the trolley problem where one or many will die, and refusing to even touch the switch is still making a choice. Being that you can’t avoid choosing, why would you deliberately choose for the worse?
@Dude
Rejecting the premise goes further than accepting the default solution. For example:
>My phone keeps running out of charge! What can I do?
>Default: “Dunno lol, just accept it”
>Reject: “Why are you on your phone so much? Are there better uses of your time?”
The idea is to identify an underlying problem and solve that instead. Similar to treating a disease vs. treating the symptoms. In the context of transportation the solution could be better urban planning to minimize the need for travel, and in the context of technology it could be social programs to encourage people to interact in real life instead of via phones. Nerds tend to miss the forest for the trees and end up treating symptom after symptom; I came here to remind people of that, but I don’t think they like it!
No, that’s just diverting the issue to irrelevant arm-chair debate. You may on the same grounds reject the second premise as well. “Why do you have to talk so much” goes into a tangle of other reasons that we aren’t trying to solve, such as “Why are you employed as a telemarketer?”, or, “Why are there cities in the first place?”, and it’s turtles all the way down from there.
Suppose you did solve a more fundamental issue to avoid solving the one at hand now – then what? Notice that you simply diverted the chain of causality towards a different set of complications that arise from your choices, and then you’re left chasing after those, trying to solve the new problems you caused.
Everything is a “symptom” of something else, and the ultimate root of your problems is existence, so if you deal with that, you’ll have the only rational solution. Right?
@Dude
“Suppose you did solve a more fundamental issue to avoid solving the one at hand now – then what?”
If you solved it, then there’s no more problem. Rejoice!
The whole point of solving an underlying problem is that sometimes it’s easier or provides a more satisfactory solution than chasing down symptoms. Once you’ve solved it though, the job’s done. Go have fun doing something else, I guess.
Furthermore, the second (third, fourth…) premise is typically harder to solve than the first, because it’s trying to change a more fundamental aspect of your existence, which in retrospect is difficult without a time machine.
For example, why did our ancestors even have to move to the US where all the resources are spread so far and wide that we now have to drive three times more than the Europeans to make ends meet? Why couldn’t we have just stayed there?
>If you solved it, then there’s no more problem. Rejoice!
As if.
Besides, it’s part of good engineering to identify such cases – to look for alternative solutions. 99% of engineering is tossing around what ifs and trying to go around problems by backtracking and sidetracking, and going around in loops to end up back where you started with the original problem because it’s a longer way to go around than straight through.
What you’re doing is just blindly applying “reject the premise” as it if makes you look smarter. Especially on matters where rejecting the premise would require you to reject the opinions of so many other people who very well prefer it this way, and they’re only trying to solve this remaining issue here.
@Dude
“What you’re doing is just blindly applying “reject the premise” as it if makes you look smarter.”
Nothing blind about it; this whole conversation has been in the context of batteries, in which I have been stating that maybe instead of relying on the energy density of batteries made from rare elements, we should instead reduce our need for high-density energy storage by reducing our total energy consumption. This seems like an entirely reasonable opinion, and doing so would solve a whole host of other problems besides batteries.
You seem determined to portray me as some sort of pseudointellectual troll when in fact I have been making entirely reasonable points this whole time. The fact that “lots of people disagree” has no bearing on anything whatsoever. Plenty of people once disagreed that the earth revolved around the sun. Most of the time, you’re better off ignoring the will of the masses when trying to solve their problems, because they are the ones who caused the problem to begin with.
And there you go generalizing about engineers again. In the past did an engineer hurt or rob you or something, just kinda odd how you’ve got a vendetta against the whole group of them. I’d say you’ve got some emotional problems (along with social problems since you seem incapable of acting like an adult). It’s sad that you act like you know everything, despite lacking in maturity in the way you interact with others. Also not sure how impressive you think you come across with a username like “anonymous”.
I’m free to generalize about whomever I please. I don’t really interact with engineers that much, but I have in the past and I just noticed they tend to all be the same sort of person. It’s really not that big of a deal, not sure why you’re so salty about it. Not sure why you’ve got a problem with people posting anonymously online, either. I do so because I’m not a narcissist and I don’t want to get doxxed. I don’t care about impressing anyone with my name (???) because that would be weird. Again, you seem disproportionately mad. Chill.
Really, because you are coming across as a grade a narcissist. You must be fun at parties. Anyway, feel free to waste more of your time replying to this chain in a whole lot of sound and fury signifying nothing, I’ve got my fill of playing with trolls for the day.
Ok weirdo, have a nice day.
> Not sure why you’ve got a problem with people posting anonymously online, either. I do so because I’m not a narcissist
Oh but you are. The real reason why you’re making arguments by obnoxious contradiction and playing the devil’s advocate here is that it brings you constant attention. The fact that nobody knows who you are is irrelevant to the point. You’re here to prove your virtue to yourself: how you can out-wit and manipulate all these people.
Don’t worry. We’ve all been there. You’re no Socrates, you’re just a village idiot who’s having fun setting other people’s beards on fire.
The applications are given. E.g. an electric car with >125kW and a range of >450km. Or a smartphone with na reasonably fast CPU and at least a 1080p OLED screen which has a battery life of1-2 days. Or a laptop with a decent CPU and at least a few hours battery operation.
These are no specifications out of a sci-fi movie, but todays current technology, not even the technical limits.
Why should anyone want to downgrade?
Anonymous must be a coal miner.
No, I support solar power and lead acid batteries, because both silicon and lead are abundant on earth.
That’s not a reasonable argument. It’s like saying you support replacing jet engines with hot air balloons because air is abundant.
No, it’s like saying you support replacing jet engines with hydrogen airships because hydrogen is abundant. Which I do.
Seems to me Lithium is more abundant than Lead
https://en.wikipedia.org/wiki/Earth%27s_crust#/media/File:Elemental_abundances.svg
I can’t tell if stupid or troll.
Right, I forgot “consume less” is never an option for you people. Gotta deplete those resources as quick as we can, after all.
Will you be saying “consume less” to the lead-acid off-roader who’s run out of power in Death Valley? To the heart patient whose pacemaker just failed?
Yes.
What, did you expect me to change my mind because sad things happen? Sorry, your bad judgement or faulty heart is no excuse for consumerism.
>no excuse for consumerism
A life-saving medical device is consumerism? You arguing by bad faith there, which is a sign of a confused person or a troll.
@Dude
Yes, forcing the world to constantly make shiny new plastic trash is consumerism, even if somewhere, somehow, it saves someone’s life. Survival is not the ultimate objective; what’s the point of living if the planet is a garbage heap?
energy efficiency is good direction but sometimes you just c ant replace power with efficiency we could have lighter passanger cars get rid of gizmos in them have carbon fibre chassis and so on but moving 40ft container needs power. But theres solutionwe also could instal better infrastructure to use energy without need for large storage – if we put induction loops under roads then the need for big batteries to have long range is reduced. For passanger cars doing daily commutes it won’t help but trucks going cross country/continent? It would also help to build railways to reduce need for trucks going cross continent. Then with fast trains need for airplanes is reduced – of course not in USA with its distances between cities but even then you can have corridors on coasts.
>why not go back to lead-acid batteries and just use less electricity?
Because you can’t use that much less without seriously limiting what you can do with electricity.
Point in case: the EV1 got up to 55 miles by filling up the back seats with lead acid batteries, in one of the smallest most aerodynamic production vehicles ever made. In normal daily commuter use, you would have to replace the batteries every 14-28 months like the USPS found out when they tested lead acids in postal vans. It would work terribly if at all in the cold, and you could not re-charge it in any reasonable time. That’s the best you can do with lead acid – a very expensive golf cart that seats two, and doesn’t go anywhere. It’s practically useless. That’s why they quickly upgraded to NiMH, and even that wasn’t enough.
So if your solution then is to “question the premise”, then you would simply have to say “do not drive electric cars”, or in the general case, “don’t do X if you can’t do it with lead-acid batteries”, which is not an answer to the original question. It would simply be an admission that your proposal would fail because there’s nothing to go back to – it never worked in the first place.
Just because we see, that we need more electricity and we need to use even more electricity to substitute non-renewable fossile fuels. Oil is gone, when you burn it. The lithium can be recycled, once a sufficient infrastructure for it is established.
And some people even think that humans could change the global climate with CO2. Although it is very unlikely, that we would have that power, the limited supply of fossile fuels is not only likely, but sure. So it is good to phase them out.
I think you’re badly informed. Lead is less abundant than lithium: https://en.wikipedia.org/wiki/Abundance_of_elements_in_Earth%27s_crust
Add the fact that you need a lot (probably close to an order of magnitude) more lead per kWh further weakens the argument for lead.
As for your ‘use less electricity’, I fully support your standpoint that we should ride a bike more. However, ask yourself why electric bikes only took off (in a commercial sense ;) ) with the advent of lithium batteries.
Lead acid electric bikes could have been made well before 2000, why did nobody try this? Probably because they quickly ran into the myriad of limitations of lead acid batteries, the high weight being the only the tip of the iceberg.
I’ve seen some talk about sodium batteries being superseded early on by the lithium type due to having half the capacity at the time but considering sodium is a waste product they could still be useful in stationary applications.
Yep. There are several elements that are potentially a lot more useful than lithium for stationary storage, including vanadium (with a very large range of oxidation states) which is used in vanadium redox flow batteries. I think these last a lot longer than lithium batteries and can give a lot lower overall cost over their lifetime.
https://en.wikipedia.org/wiki/Flow_battery
To me, seems the stationary locations can utilize the worse most toxic materials for safer storage since the materials and the batteries or energy storage device will have to be secured anyways.
Seems win-win to use safer and in a healthier utilitarian way.
Unless somehow turning back to rocks basically where won’t leach the toxic materials so much.
I’m actually surprised due to the abundance of sodium, sodium isn’t used more for batteries and from previous projections when I researched briefly a few years back… they’d be on the market by now.
I’ve wondered about alloys use also, though I’m no battery expert.
I would not consider sodium as a waste product. It was always said, that the chlorine is the waste product from sodium and caustic soda production.
But you are right in that way, that sodium is very abundant – around 3% NaCl in seawater.
The paucity of Lithium is due to how heavier elements are created: most happen in the dying days of the largest stars where heavier and heavier nucleii are fused together. The Hydrogen (1 proton) is mostly gone so you see fewer odd-numbered-protons nucleii than evens which are made by multiples of Helium (2 protons).
Hey, if I were you, I wouldn’t carry a lithium battery in my pocket, that’s just unsafe, Matthew.
(I realize you mean in a phone, I just had nothing of value to add, so like some of the other commenters, I decided to nitpick instead. Its still solid advice though, I’m surprised I haven’t burned myself badly by absentmindedly tossing 18650 or 20700 cells in pockets.)
For fixed and in particular large scale energy storage the most appropriate technology may be the Iron-Chloride Redox Flow Battery because iron is one of the most abundant elements on Earth and we should expect an excess of chlorides from the extraction of other elements from chloride salts. Lithium should only be used where it is essential.
How many processes are involved in just extracting lithium from where ever, transporting it to where ever, refining it using lord knows what chemical processes, when done with product, what processes and chemicals/infrastructure is needed to even recycle this stuff, which will have to be mandatory. On the back of child and or other expolited labor, all the while ravishing whatever country is unfortunate enough to have a stockpile of lithium. Lithium shortage? A good excuse to raise the price of an already expensive proposition, as there is little used car market, so everyone has to buy a new electric vehicle? And just how is the used electric car market going to even work? Sounds like all cars are going to have to be leased to make this garbage work, sign me right up. I mean seriously, child labor, but nothing to see here because smart phone and electric cars, in the same sentence. All a joke, and a very bad one at that. Just wait for self driving to come around, going to save the planet with giant remote control cars, best science has to offer. Edison would be proud there has been advancement in the ancient battery.
It’s just more shiny plastic to keep the nerds happy. They run the place now, so everyone else has to shovel rocks to build their new toys.
Go move yourself “into the woods” if you want to go back to the stone age (or btw. “lead age” of batteries), but leave the other people their lifestyle. That’s the problem with all that left-extreme environmental hysterisc: They want to force their idea of life onto other people.
I do not really understand your rant, what in it is serious, what is joke.
What do you want? Stay with conventional gasoline engine? There are also many processes involved in the production of gasoline.
Self driving car should not be “remote control” but an intelligent computer IN the car. And not to much external “connectivity”. I do not want a tracking device which can not be opted out.
Ford, Edison as well.
When you get older, the eyes takes longer to adapt between dark and light, which makes hackaday harder to read.
However, I’ve used many different kind of (real) terminals during the years, green or white text on black and black on white, and even then I still preferred black text on white background.
I assume that black background is cooler than white since it looks like an old terminal from 1980, but I think it should be possible to choose which theme suits you the best.
Oops, wrong window…
But true.
>Several have compared these market conditions to the oil industry. The demand for oil led to new methods for extraction and new technology that weren’t commercially viable before.
The oil boom happened because people were suddenly willing to pay for it, because they found it contained so much energy and they could use it for a million things that were much more expensive without it – so more investments kept flowing in, so more oil was extracted. Oil prices didn’t go down, they went up, which fueled the rapid expansion in the industry. The 1860’s first oil rush saw prices in the $120 a barrel in today’s money.
For lithium the market is different, because it’s not a technological trigger like the discovery of oil and all its properties. We already have cheaper and better solution to the problem that we are simply trying to substitute with lithium batteries, because of some half-baked political idea that we must do it by hook or by crook – so it’s a waiting game to get the prices down enough that we can actually do it.
In other words, the demand for batteries will stall if the price doesn’t keep coming down, but the industry can’t expand if the prices won’t go up. If Mohammad will not come to the mountain, the mountain must therefore come to Mohammad, apparently.
> Cathodes are often made from lithium nickel cobalt (LMO) or Lithium nickel manganese cobalt oxide (NMC).
Did I find the hidden typo?
https://www.nytimes.com/2010/06/14/world/asia/14minerals.html
I thought it was a song by Nirvana… Ok, I see myself out.
If it’s so light and such a simple element, it will be one of the first elements that we’ll be able to synthesize in the future.
Will not beeconomic, unless it would be literally a waste product vom nuclear fusion reactors. But unfortunately the opposite is much more likely: lithium will be necessary to breed tritium for fusion reactors.
Came to say that lithium (bicarbonate
) saved my dad’s life continually from the time he started taking it (one of the first in the USA, I was told) until some nitwit convinced him to go off it in the 90’s (another story).
Thank you to everyone positively involved in making this a viable treatment for bipolar disorder.
interesting read. The real breakthrough in electric world comes when someone finds a way to viably store electric charge into something else than a chemical reaction. Supercaps store the energy into electric field and superconductive coils into magnetic field. Maybe a quantum trap emitting radiation converted to current?
I wonder if it would be a good investment to charge for “recycling” used cells, store them for couple of decades and sell as a raw material for a profit?
Mental note: Visit Uyuni ASAP.
The way shit’s going we’ll be mining Lithium from asteroids for it’s antidepressant value.
This article seems like a great place to plug some advocacy work for solar concentrating trough systems for sea water processing for desalination, mining, radar installations and more border security. I’m sure the later aren’t so popular for some if not many. Desalination and mining for certain with some electricity production while at then.
Maybe a plug for deep sea high pressure contained external combustion on the geothermal vents with or without thermonuclear submarine power plants to process sea water for the above said uses. Come on, a fricken Tesla in space? What the practical flipping why the fricken not?
Seems to me Lithium is more abundant than Lead
https://en.wikipedia.org/wiki/Earth%27s_crust#/media/File:Elemental_abundances.svg
Apologies, this reply ended up in the wrong place.
If we have enough to build a billion batteries, then there isn’t enough. There are 8 billion people.