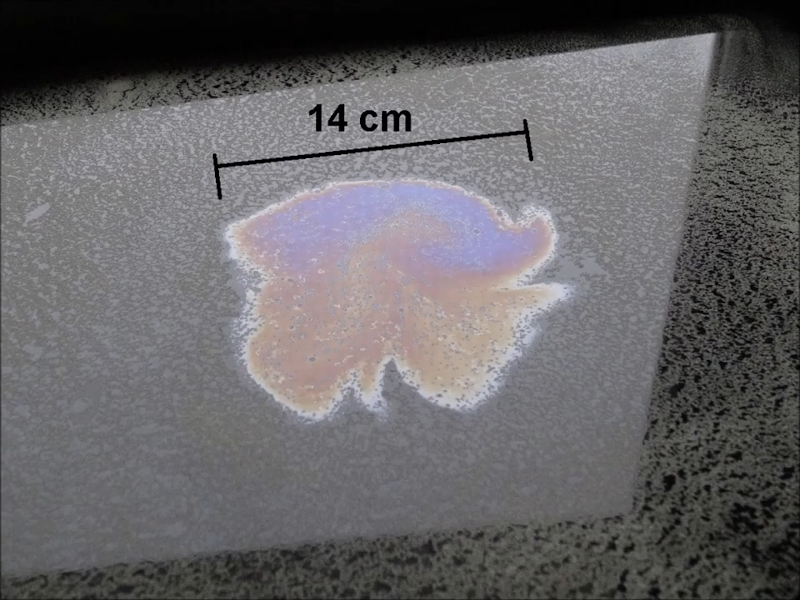
Do you need a well-equipped lab to measure the size of an atom (German, machine translation)? According to [stoppi], no. You need sunflower oil, some bear moss spores, and a bit of gasoline. You’ll also need some common things like a syringe, a baking sheet, and a jar. You can see the whole process in the video below. The measurement isn’t really for a specific atom, but it is an average for a lipid molecule, which is still impressive.
You essentially measure the diameter of an oil drop spread over water. Since the oil is mostly oleic acid, the height of the layer is known as 167 atoms. After that, it is some simple measurements and math to get the height and find the average atom height.
The measurement relies on the fact that oleic acid is a lipid and is part hydrophobic and part hydrophilic. This means that when an oleic acid molecule hits water, it stands in a particular orientation. A drop of oil will spread to a single molecular thickness. If you know the volume of oil you introduced, you can mathematically deduce the height of the spread out oil drop by considering that the volume of the disk must be the same as the volume you started with.
While your mileage may vary, [stoppi] got 1.76 angstroms by taking the height of the film and dividing by the number of atoms in the film. That’s 176 picometers per atom. If you can’t find spores, talcum powder is known to also work in this application and you can use alcohol instead of gasoline, too. You might find it handy to put the powder in a salt shaker to distribute it. You’ll want to tap the tray to make sure the oil drop spreads as far as possible. It is also a good idea to take an average diameter measurement since your oil drop is likely not going to be perfectly circular.
The process isn’t new. In 1932, [Irving Langmuir] picked up a Nobel prize for work that included this method. If you’d rather see your molecules, try 3D printing. If you are a fan of measuring the physical universe, why not try finding the speed of light?















https://youtu.be/lmgCgzjlWO4
This looks A LOT like Matt Parker & Steve Mould’s experiment with oleic acid for Pi Day 2021. For their experiment, they worked backwards, using the volume of an oleic acid molecule and a couple known constants to calculate pi. Here, they worked forward with pi and a couple of other known constants to derive the volume of an oleic acid molecule (and used some back-of-the-envelope approximations to get the diameter of an atom)
I remember this one from high school physics (UK, late 80s). The teacher assured us that nobody in the world has ever had an allergic reaction to the spores before and we don’t have to worry, which left me far more worried about the spores.
Try not to think of polar bears for five minutes.
We did this in Higher Physics in 1986-7. Measuring the diameter of the oil spot is a lot more difficult when Neil Roberts comes along holding a ruler which he suspends a centimetre above your basin and says loudly that WOULDN’T IT BE A SHAME if he stirred it? In the resulting struggle our oil got thoroughly stirred and the measurement was lost.
The spores we used when performing this experiment in 60s high school chemistry class were lycopodium powder.
“You need sunflower oil, some bear moss spores, and a bit of gasoline. You’ll also need some common things like a syringe, a baking sheet, and a jar. ”
Sounds like something MacGyver would use.
You need to know that the height of the layer is 167 atoms.. But that height is derived of the atom measure!
How to measure the radius of the sun? You only need a calculator! Since the sun is known to have a diameter of 1.392 million km, you only need to divide that by 2.
You can count the number of atoms through other means. For instance by measuring the volume of CO2 it produces during combustion.
HaD should get edit buttons for the comments.
Here’s a page on combustion analysis: https://preparatorychemistry.com/Bishop_Combustion_Analysis.htm
This oil film experiment was done in 1917. Combustion analysis relies on gas chromatography which came out much more recently. ” German physical chemist Erika Cremer in 1947 together with Austrian graduate student Fritz Prior developed the theoretical foundations of [gas chromatograaphy] and built the first liquid-gas chromatograph, but her work was deemed irrelevant and was ignored for a long time.”
So, once again, you are using much more recent techniques and equipment to alter the meaning and significance of results decades earlier. It would be like apply a technique that wont even be theorized until the year 2060 to the work being done at CERN today and then pretending the work CERN is doing now is much less significant and has a totally different meaning than it really does.
gas chromatography is not needed. You can just burn a given amount of the compound, and absorb the resulting CO2 in a solution of lye. The weight increase of the solution is easily measured.
Similarly, you can condense the H2O, weigh it, and deduce the amount of hydrogen in your compound.
etc. etc.
The article is interesting, your comment makes it laughable.
Read my replies to Jose and you’ll see that you might have come to the laughing conclusion a bit to fast.
Knowing the number of atoms in a compound is not the same as knowing their size.
I remember doing a similar experiment at the request of my physics teacher to prove the new Nuffield science project that was going to be taught from the beginning of the following year. We derived a molecular diameter within a factor of 10 of the value in the reference book. Actually quite close, I believe.
We started off by preparing a baking tray with wax and carbon black to make a background against which we would be able to see the slick, then filled it with water to make a positive meniscus. The oil drop was measured, dropped in and the diameter of the slick measured, then the volume assumed to be the same as in the German experiment description except we assumed the film to be one molecule thick.
The tray preparation took a little time, but could have been done prior to the lesson. Apart from this it seems to be a much simpler experiment than mixing chemicals and throwing in a ‘magic number’ to make the sums work.
I was most impressed with the simplicity of the experiment and accuracy of the result, so much so that it is still memorable today, over half a century later.
Atom Molecule … Typical HaD title FAIL.
This makes a lot of semi-valid assumptions based on much more complicated work done by much smarter people with much more expensive equipment.
How do you known sunflower oil is most oleic acid, what does “mostly” mean, and how does it effect your result? How do you know a drop of oil on your fluid of choice will spread to a single molecule thick? How do you know the layer height is 167 atoms thick? What does that mean, hydrogen atoms? carbon atoms? Einsteinium atoms?
Irving Langmuir won his Nobel prize for determining the thickness of the molecule which gave insight into the molecular configuration of the oil. His work was in surface fluids and he showed that the process would determine the thickness of a molecule. If they already had spectroscopic techniques to know how thick the molecule was in atoms (which is how they got your 167 atom thick figure), Mr. Langmuir’s work would have been as pointless as this entire article/project.
On a related note. I’ve devised a method to measure the most common isotope on earth of every element. Just look up the atomic mass listed on a periodic table, round to the nearest whole number and that’s the most common isotope. So simple and elegant, where’s my Nobel prize?
I didn’t read the article but judging from the picture 14 cm is a big damn atom!
167 atoms? Why? I’m not sure the number of atoms in the molecule is a measure of the ‘height’ dimension of the molecule. If the three oleate units are aligned together, then wouldn’t the ‘height’ of the thing be determined more by how many carbon-carbon bonds (and a few carbon oxygen bonds) there are between the glycerol unit and the end of one of the oleate units, assuming that the molecule ‘stands up’ in the monolayer? (the linked page shows the glycerol trioleate rather than oleic acid as the molecule being used).